Abstract
The rat retrosplenial cortex (RSC) makes critical contributions to learning and memory but these contributions may not be uniform along its rostro-caudal axis. Previous work suggests that event-related and context-related information are differentially encoded by anterior and posterior RSC subregions. Here, we further test this idea using a procedure in which spatial/environmental cues (context) and discrete event memories are acquired separately. All animals received a 5-min pre-exposure to the training context 24 h before contextual fear conditioning where shock was delivered immediately upon being placed in the chamber. Rats were tested for memory for the context the next day. We found that optogenetic inhibition of cells in only the posterior RSC during the pre-exposure phase, when spatial information is encoded, reduced behavioral responding during the subsequent memory test. However, similar inhibition of either the anterior or posterior RSC during shock delivery, when information about both the context and the shock become integrated, impaired memory. Finally, inhibiting cellular activity in only the posterior RSC during memory retrieval during testing reduced responding. Together, these results suggest that while activity in both subregions is needed during the period in which the event-related information becomes integrated with the context representation, the posterior RSC is important for both memory formation and retrieval or expression of memory for information about the context. These results add to a growing literature demonstrating a role for the RSC in integration of multiple aspects of memory, and provide information on how spatial representations reliant on the retrosplenial cortex interact with associative learning.
Keywords: context, learning, memory, retrieval, retrosplenial cortex
Introduction
Memory formation often involves learning not only about discrete events and stimuli, but also about the surrounding spatial cues or context in which those events and stimuli are presented. Failures to bind multiple types of information to appropriately contextualize learning are symptomatic of several neuropsychiatric diseases, ranging from post-traumatic stress disorder to Alzheimer’s disease. It has been suggested that the retrosplenial cortex (RSC) is an important structure for integrating multiple aspects of memory during consolidation (Todd and Bucci 2015; Miller et al. 2019, 2021). In line with its role in information integration, the retrosplenial cortex is important for memory of complete spatial environments (Harker and Whishaw, 2002; Vann et al. 2003; Cain et al. 2006; Miller et al. 2019, 2021), as well as for context memory in both contextual (Keene and Bucci 2008a, 2008b; Robinson et al. 2012; Todd et al. 2017; Yamawaki et al. 2018) and trace (Kwapis et al. 2014, 2015; Trask et al. 2021a) fear conditioning paradigms.
Some evidence suggests that contextual memory might be more reliant on the posterior region of the RSC. While damage to the posterior RSC (pRSC) impairs spatial memory (Vann et al. 2003), selective damage to the anterior region (aRSC) does not (Neave et al. 1994; Vann and Aggleton, 2002). In contrast to the pRSC, the anterior RSC has been implicated in memory for discrete stimuli (de Landeta et al. 2020). Together, these data suggest that the contributions of the RSC in processing specific elements of a memory are not uniform along its rostro-caudal axis and that neural activity throughout the retrosplenial cortex is needed to support integration of multiple aspects of memory. We have recently begun to investigate the role of discrete retrosplenial subregions in encoding different aspects of memory. We used optogenetics to inhibit neural activity during training trials in trace fear conditioning, which involves learning information both about the conditional stimulus that predicts shock as well as about the context in which training occurs (Chowdhury et al. 2005; Kwapis et al. 2015). We found that inhibiting neural activity in the anterior RSC impaired later memory for a discrete stimulus but left context memory intact. The opposite pattern was observed when we inhibited activity in the pRSC in that memory for the context was impacted but there was no impact on auditory stimulus memory (Trask et al. 2021a). These results suggest that the anterior portion of the RSC may encode information about discrete events while the pRSC has a separable role in encoding spatial information. We (Trask et al. 2021a) and others (Todd and Bucci 2015; Miller et al. 2021) have hypothesized that the retrosplenial cortex might be especially important in binding simultaneous stimulus elements during memory acquisition.
One interesting finding in contextual fear conditioning is that animals that receive shock immediately when placed in the chamber show reduced freezing during long-term (e.g., 24 h) retention tests (Fanselow 1986; Landeira-Fernandez et al. 2006). This “immediate shock deficit” can be alleviated, however, if the animal has received brief exposure to the conditioning environment prior to the conditioning episode. Procedures that interfere with memory consolidation during this context pre-exposure phase eliminate the ability to learn normally at training. This suggests a critical role for successful context encoding before it can be associated with an aversive unconditional stimulus or UCS (Cullen et al. 2017). This rescue of the immediate shock deficit by exposure has been called the context pre-exposure facilitation effect (CPFE) and depends on forming a coherent, unitized (i.e., fully integrated) representation of the spatial environment in which learning will take place and then later integrating this information with knowledge of the shock (Fanselow 1986), suggesting a potential role for the retrosplenial cortex.
The predicted role of the RSC in the spatial learning central to the CPFE has begun to receive empirical support. In one report, Todd et al. (2017) demonstrated that while pre-training lesions of the entire RSC had no impact on the CPFE, post-training lesions of the RSC reduced freezing relative to controls. Importantly, this paradigm can be separated into two discrete phases, capturing both the “where” (i.e., context) and “what” (i.e., event) components of associative learning. As such, the context pre-exposure facilitation effect provides a novel and unique opportunity to further disentangle the contributions of the retrosplenial subregions to distinct aspects of aversive memory formation and information binding necessary for successful memory formation and retrieval. We hypothesized that the posterior retrosplenial cortex is important for the encoding of context-related information whereas the anterior retrosplenial cortex is important for the encoding of event-related information.
In order to test this hypothesis, we first examined expression of the immediate early genes zif268 and c-fos in both the anterior and posterior RSC following pre-exposure, foot shock delivery, or memory testing using the context pre-exposure facilitation procedure. Zif268 expression was chosen because it has a well-known role in associative memory formation and can also serve as a general marker of neural activity (Ferrara et al. 2019). Furthermore, Asok et al. (2013) reported elevated zif268 mRNA in the retrosplenial cortex following both the pre-exposure and immediate shock phases of the context pre-exposure facilitation effect paradigm. We also examined the immediate early gene c-fos, which has been closely linked to neuronal activity associated with fear memory retrieval (Rajbhandari et al. 2016; Keiser et al. 2017). Our primary goal in this experiment was to map how IEG expression changed within the same animal along anterior–posterior axis of the retrosplenial cortex following each discrete phase of memory encoding and retrieval. We then used optogenetics to inhibit activity during either the pre-exposure, shock, or testing phases in either the anterior or posterior RSC. We have recently demonstrated that optogenetic inhibition of the aRSC or pRSC results in a selective depression of zif268 expression in the targeted region without impacting zif268 expression throughout the RSC (Trask et al. 2021a). We predicted that the pRSC would be uniquely important for the pre-exposure phase, the aRSC would be uniquely important for the shock phase, and that both would be necessary for subsequent memory retrieval and freezing during thetest.
Methods
Subjects. Male Long-Evans rats (300–400 g; Harlan, WI) were housed individually in plastic cages with chip bedding and free access to food and water, in accordance with the University of Wisconsin-Milwaukee Institutional Animal Care and Use Committee. The room where animals were housed was maintained on a 14:10 light/dark cycle.
Surgical Procedures. Virus infusion surgeries proceeded exactly as described in Trask et al. (2021a). Solution containing AAV9-CAG-ArchT-GFP or AAV9-CAG-GFP recombinant virus (obtained from the University of North Carolina Vector Core; titer: 2 × 1012 molecules/ml) was infused at multiple sites in either the aRSC or pRSC. This virus causes expression of a light-activated proton pump in all cell types throughout the targeted region (including neurons). Rats were anesthetized with isoflurane and placed in a stereotaxic frame. Six 0.5-mm diameter holes were drilled in the skull above either the aRSC or pRSC. Coordinates for the anterior infusions were 0.5 mm lateral, 1.8 mm ventral, and 1.6, 2.6, and 3.6 mm posterior with respect to bregma. Coordinates for the posterior infusions were 1.0 mm lateral, 1.8 mm ventral, and 5.6, 6.6, and 7.6 mm posterior with respect to bregma. Using a 10-μl syringe and a 34-gauge needle (World Precisions Instruments, Sarasota, FL), 0.3 μl of either ArchT or control virus was injected at a rate of 50 nl/min. Coordinates were chosen based on previous findings demonstrating that selective lesions of the pRSC reduce spatial learning (Vann et al. 2003) but more anterior lesions have no impact (Neave et al. 1994), as well as on our own work using the present method to demonstrate dissociable roles for the aRSC and pRSC in encoding distinct aspects of a trace fear memory (Trask et al. 2021a). The needle was left in place for an additional 10 min to allow for diffusion away from the injector. This was repeated once at each of the six injection sites for each animal. The incision was sutured and each animal was given six weeks to allow for optimal expression of opsins.
Following the six-week recovery period, LED implants were mounted to the skull above the infusion site for each rat using a procedure similar to Cowansage et al. (2014) that we have previously employed (Trask et al. 2021a). The skull was thinned to create a 2-mm2 translucent area centered above the infusion sites. Silicon-encased, prewired surface-mount 5050 trichip ultrabright LEDs (Green-521 nm; oznium.com) were affixed with clear superglue centered over the skull window. Encased LEDs were secured to the skull with two screws, cyanoacrylate, and dental cement. Rats were allowed 3–5 days of recovery following LED implantation prior to behavioral procedures.
Behavioral Procedure. Following recovery from LED implantation, animals were placed in a Med Associates (St. Albans, VT) conditioning chamber (30.5 × 24.1 × 29.2 cm) housed in individual sound attenuating chambers. Chambers were illuminated with an incandescent house light and exhaust fans provided a 65-dB background noise. A scent was created by cleaning each chamber with 100% ethanol immediately before the animal was placed inside. Rats were left in the chamber for 5 min. The next day, animals were given 5 footshock UCS presentations immediately upon being placed in the chamber. The UCS was a 1-s 1 mA footshock. The ITI between each UCS was 1 s. Animals were removed immediately following the final footshock. On the final day, contextual fear retention was assessed by measuring freezing in the original conditioning chamber in the absence of any shocks for 5min.
Freezing was defined as the cessation of all movement excluding respiration and was automatically scored in real-time with FreezeScan 1.0 detection software (Clever Sys, Inc.) calibrated to a trained human observer. In order to reduce variability in freezing observed with animals attached to the patch cord at different phases in the experiment, behavior for aRSC and pRSC groups was calculated by calculating the freezing as a percentage of control animals who were given the same surgical and behavioral procedures, except that when they were attached to the patch cord for optogenetic manipulations no light was delivered. This calculation puts all of the behavior all on the same scale without altering the pattern of observed results between aRSC and pRSC groups given the same treatment.
Light Delivery. LEDs were controlled via TTL pulses from a computer running Med Associates software (Med Associates, St. Albans, VT). Rats were connected to a patch cord and placed in the chambers at the beginning of the training session. Continuous light activation (5 mW) began as soon as the animal was hooked to the patch cord and placed in the chamber and remained on for the duration of either the pre-exposure, training, or testing periods. This created 6 experimental groups by parametrically manipulating brain region of inhibition (anterior or posterior RSC) with experimental phase (Pre-Exposure, Shock, or Testing).
Histology. At the end of each experiment, rats were sacrificed and brains were sectioned to verify the extent of the virus infusion. For sagittal sectioning, brains were frozen and mounted on charged glasses slides. Slides were cover slipped with Fluoroshield mounting media (Sigma) and images of GFP-expressing cells were obtained with an Olympus FV1200 confocal microscope to verify the extent of virus expression. Representative tissue images and extent of virus expression are depicted in Figure 3. Notably, there was no overlap between aRSC and pRSC regions and ArchT expression was restricted to the target region in all but one animal, who was consequently removed from analyses.
Figure 3.
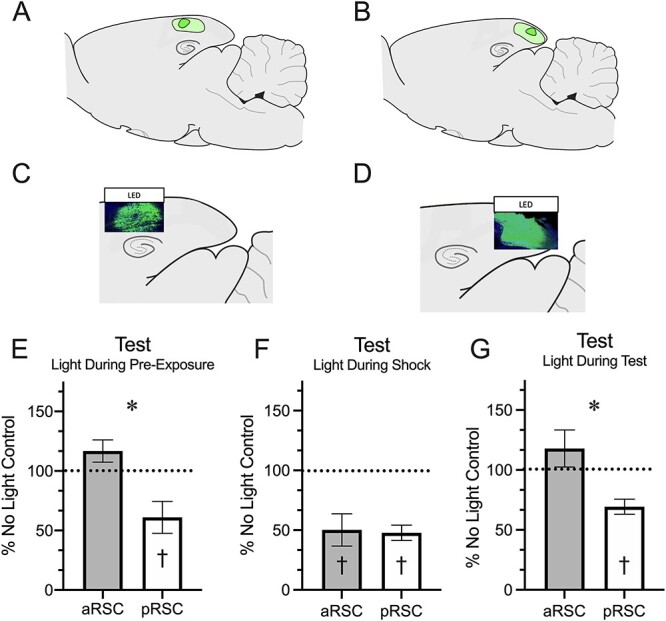
Inhibition of the anterior or posterior RSC during the distinct phases of the context pre-exposure facilitation effect has differential impact on memory during context testing. (A) Minimum (dark green) and maximum (light green) spread of virus in the aRSC. (B) Minimum (dark green) and maximum (light green) spread of virus in the pRSC. (C) Representative image of green fluorescent protein in the aRSC. (D) Representative image of green fluorescent protein in the pRSC. (E) Freezing during the test following LED activation during pre-exposure. (F) Freezing during the test following LED activation during shock. (G) Freezing during the testing period during which the LED was activated. * = P < 0.05 between aRSC and pRSC. † = P < 0.05 from the No Light condition.
Immunofluorescence. For the immunofluorescence experiment, rats were sacrificed 60 min following either context exposure, training, or retrieval testing to quantify zif268 and c-fos expression associated with stimulus-related neural activity (Lee et al., 2005; Ferrara et al. 2019). Brains were immediately removed and stored at −80°C until sliced in 20-micron coronal sections and mounted onto charged slides. Slides were rehydrated in wash buffer (PBS + 0.05% Tween-20) and permeabilized (PBS + 0.3% Triton X) for 15-min and incubated in blocking solution (PBS + 0.7% NGS). Slides were then incubated in zif268/EGR1 primary antibody (Cell Signaling, 1:500, #4153) or c-fos primary antibody (Santa Cruz, 1:100, sc-52) solution (PBS + 0.3% Triton X + 5% NGS) overnight at 4°C. The next day, slides were incubated in secondary antibody solution (Thermofisher: Alexa Fluor 594, 1:500) for 2 h and rinsed with wash buffer, a DAPI counterstain was applied, and slides cover slipped. Images were captured on the Olympus Fluoview FV1200 confocal microscope using a 40× objective lens. Serial z-stack images covered a depth of 4.55 μm through five consecutive sections (0.91 μm per section) and were acquired using Fluoview software (Olympus). Midsagittal slices were collected from each animal. Three to six images from the anterior and posterior RSC were taken for each animal (with exceptions being made for damaged tissue), giving a within-subject measure of activity along the anterior/posterior axis. Images were taken from −2.60 mm and − 6.60 mm relative to bregma (see Fig. 1). zif268 and c-fos expression (total particle counts measured using the “Analyze Particles” plugin in ImageJ) were normalized as a proportion of total amount of DAPI particles present on the same sampled section.
Figure 1.
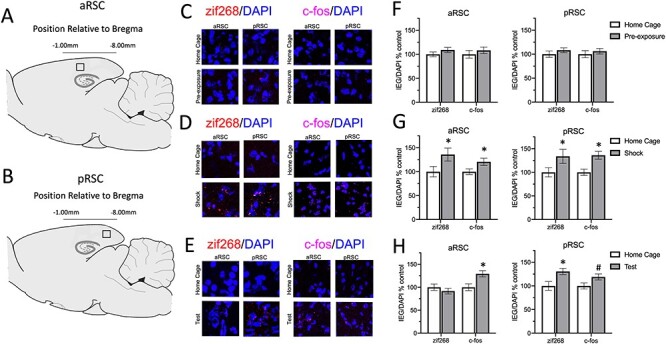
Different phases of the context pre-exposure effect are associated with distinct patterns of IEG activity throughout the RSC. (A) Sagittal view of the rat depicting where images were taken from each slice in the anterior retrosplenial cortex (aRSC). (B) Sagittal view of the rat depicting where images were taken from each slice in the posterior retrosplenial cortex (pRSC). (C) Representative images of zif268 (left) and c-fos (right) staining taken at 40× from each group from the aRSC and the pRSC. The top row represents images from the same slice taken from an animal in the control group; the bottom row has images taken from the same slice from an animal who was sacrificed after pre-exposure. (D) Representative images of zif268 (left) and c-fos (right) staining taken at 40× from each group from the aRSC and the pRSC. The top row represents images from the same slice taken from an animal in the control group; the bottom row has images taken from the same slice from an animal who was sacrificed after shock. (E) Representative images of zif268 (left) and c-fos (right) staining taken at 40× from each group from the aRSC and the pRSC. The top row represents images from the same slice taken from an animal in the control group; the bottom row has images taken from the same slice from an animal who was sacrificed after testing. Panels (F-H) Quantified expression of zif268 and c-fos expression as a proportion of DAPI on the same slice (normalized to the control group) following pre-exposure (F), shock (G), or testing (H). * = P < 0.05, # = P < 0.10 between control and experimental conditions.
Statistical analysis. All results were analyzed using either one-way or repeated measures analyses of variance (ANOVAs) or t-tests using SPSS (Statistical Package for Social Sciences; IBM) software, with alpha set to 0.05. Planned comparisons were conducted to examine between-and within-group differences following significant main effects or interactions. One animal from group pRSC was excluded for being a statistical outlier (Z = 2.07; Field 2005) and one for a lack of virus expression. Final group sizes for the immunofluorescence experiment were: Pre-Exposure (PE): 6, PE Control: 4, Shock (S): 6, S Control: 4, Testing (T): 6, T Control: 4. Final group sizes for the behavioral phase experiment were: Pre-Exposure aRSC: 9, Pre-Exposure pRSC: 6, Pre-Exposure No Light: 8, Shock aRSC: 9, Shock pRSC: 11, Shock No Light: 6, Test aRSC: 5, Test pRSC: 7, Test No Light: 7.
Results
IEG Expression Patterns Support Unique Roles of the Retrosplenial Subregions in Memory Formation
Results from the IEG expression experiment are depicted in Figure 1. Each group was compared to a group who had been treated identically up to the day that they were sacrificed. On these days, control animals were left in the homecage during the time in which the experimental group received the behavioral procedure. A 3 (Sacrifice Phase: Pre-Exposure, Shock, Test) x 2 (Group: Experimental, Home Cage) × 2 (Brain Region: aRSC, pRSC) ANOVA was conducted to assess zif268 expression following pre-exposure, shock, or testing. This found a main effect of group, F(1, 131) = 7.13, P = 0.003, and a three-way interaction, F(2, 131) = 2.97, P = 0.055, and a region by experiment interaction, F(2, 131) = 2.91, P = 0.055. No other main effects or interactions approached significance, largest F = 2.42, P = 0.11. Because there was a main effect of group, planned comparisons were conducted to compare each experimental group to their respective control in both brain regions. Pre-exposure to the context did not result in a substantial increase in zif268 activity in either the aRSC or pRSC, Fs < 1 (Fig. 1F). The shock procedure increased zif268 expression in both the aRSC, F(1, 131) = 7.56, P = 0.007, and pRSC, F(1, 131) = 5.80, P = 0.017 (Fig. 1G). Following testing, while there were no differences in the aRSC, F < 1, zif268 was significantly elevated in the pRSC, F(1, 131) = 4.53, P = 0.035 (Fig. 1H). When examining differences between aRSC and pRSC, no within-subject differences were found following either pre-exposure or shock, Fs < 1, there was significantly elevated zif268 expression following testing in the pRSC than the aRSC, F(1, 131) = 20.92, P < 0.001.
The same 3 (Sacrifice Phase: Pre-Exposure, Shock, Test) × 2 (Group: Experimental, Home Cage) × 2 (Brain Region: aRSC, pRSC) ANOVA was conducted to assess c-fos expression. This found a main effect of group, F(1, 126) = 14.42, P < 0.001, but no other main effects or interactions, largest F = 1.70, P = 0.19, indicating that while experimental groups had more c-fos expression than home cage controls, this did not differ by brain region. In order to examine the main effect of group, planned comparisons were conducted to assess if animals differed from home cage controls following each phase of the experiment. These planned comparisons found that animals did not show increased c-fos relative to controls in either the aRSC or pRSC following pre-exposure, Fs < 1, but c-fos was increased following shock both the aRSC, F(1, 126) = 4.09, P = 0.045, and the pRSC, F(1, 126) = 13.19, P < 0.001. Following testing, c-fos was elevated in the aRSC, F(1, 126) = 6.71, P = 0.011, and slightly elevated in the pRSC, F(1, 126) = 2.97, P = 0.087.
Because we saw differences based on phase in zif268 expression, we further examined within-subject differences between activity in the aRSC and the pRSC. A difference score was calculated for each zif268 and c-fos observation (subtracting activity in the aRSC from the pRSC). We found that while control and experimental groups did not differ in zif268 expression on this metric following either pre-exposure (Fig. 2A) or shock (Fig. 2C), ts < 1, they did following testing (Fig. 2E), t(42) = 3.18, P = 0.003. No differences were observed in c-fos expression between the aRSC or pRSC at any point (Fig. 2B, D, F), largest t(43) = 1.70, P = 0.10. Together, these results show differential expression of zif268 in the aRSC and pRSC during retrieval of a contextual fear memory.
Figure 2.
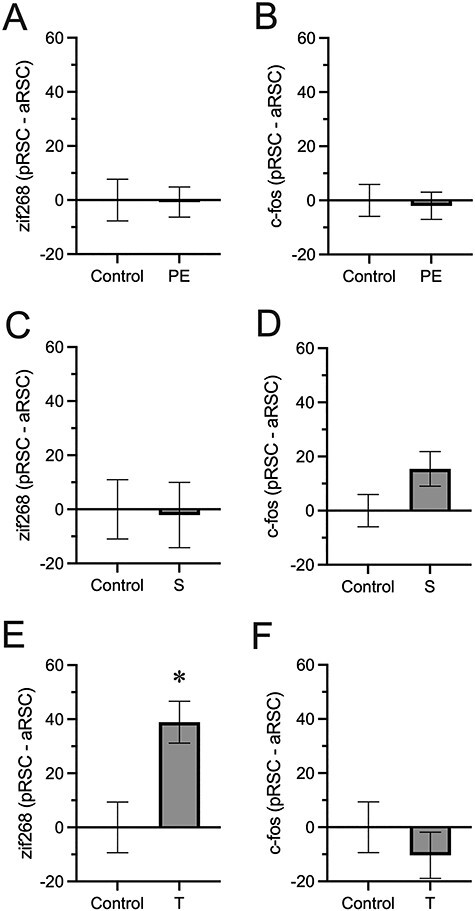
Difference scores capturing changes in IEG expression along the rostro-caudal axis of the RSC following pre-exposure (PE), shock (S), and testing (T) relative to a home cage control. Difference scores were taken by taking normalized IEG expression values (Fig. 1) and subtracting expression in the aRSC from pRSC expression. (A) Difference scores in zif268 expression following pre-exposure. (B) Difference scores in c-fos expression following pre-exposure. (C) Difference scores in zif268 expression following shock. (D) Difference scores in c-fos expression following shock. (E) Difference scores in zif268 expression following testing. (F) Difference scores in c-fos expression following testing. * = P < 0.05 between control and experimental conditions.
Memory Formation and Retrieval Are Disrupted by Subregion-Specific Inhibition of the Retrosplenial Cortex
A 2 (Region: aRSC, pRSC) × 3 (Inhibition Phase: Pre-Exposure, Shock, Test) ANOVA conducted to assess responding during the testing phase. This found main effects of both region, F(1, 41) = 15.87, P < 0.001, and phase, F(2, 43) = 11.49, P < 0.001, and an interaction between the two, F(2, 41) = 4.09, P = 0.024. Planned comparisons were conducted to compare brain regions across each phase. Following inhibition during pre-exposure, groups differed during testing, F(1, 43) = 8.05, P = 0.007 (Fig. 3E). Furthermore, inhibition of the posterior t(5) = 2.91, P = 0.03, but not anterior, t(8) = 1.80, P = 0.11, RSC during pre-exposure resulted in suppressed freezing to the context during testing. aRSC and pRSC inhibition during the shock exposures did not have a differential impact on freezing during testing, F < 1 (Fig. 3F), but inhibition of either aRSC, t(8) = 3.68, P = 0.006, or pRSC, t(10) = 8.14, P < 0.001, reduced freezing. Inhibition during testing had a similar impact on responding during the test as was observed following inhibition during pre-exposure with the pRSC group freezing less than the aRSC group, F(1, 41) = 7.86, P = 0.008 (Fig. 3G). Again, only the posterior group showed a suppression in freezing t(6) = 4.87, P = 0.003, with silencing of the aRSC having no impact, t(4) = 1.16, P = 0.31.
Discussion
We found that simple exposure to the context did not alter zif268 or c-fos expression in either the anterior or posterior RSC, while training with an immediate shock resulted in increased zif268 and c-fos expression in both regions. During memory testing, in which memory for context-shock relationship should be retrieved, c-fos was elevated in both regions but only the posterior RSC showed elevated zif268 expression. We then demonstrated that optogenetic inhibition of the pRSC during pre-exposure, shock, or testing reduced freezing to the context during testing demonstrating a selective role for the pRSC in supporting contextual information during memory formation and retrieval. However, similar inhibition of the aRSC only impacted later performance if it was applied during the shock phase. aRSC inhibition during the context encoding or retrieval phase had no impact on performance. Together with the IEG results and in line with prior results, this demonstrates that the more anterior region of the RSC is not necessary for spatial memory but is needed for the context to become associated with an aversive outcome.
These results extend the work of Todd et al. (2017) in understanding the contributions of the retrosplenial cortex during the context pre-exposure facilitation effect paradigm. As mentioned above, they found that while pre-training lesions of the entire RSC did not affect freezing to the context, post-training lesions of the RSC did. Their results suggest that when training and retrieval occurs in the absence of any RSC activity other brain regions can compensate for a lack of RSC activity but when training occurs with RSC activity, memory becomes reliant on this region for later expression. However, in our results, temporally precise inhibition of the pRSC during pre-exposure or of either subregion during shock reduced later freezing to the context. This suggests that complete lesions several days prior to training might allow for other regions to compensate for the lack of RSC activity to produce the CPFE, but a more limited (either 5-min or 11-s) inhibition of activity might not allow for the same degree of compensation.
Recall that Asok et al. (2013) found increases in zif268 expression following mere pre-exposure to the context, a result that was not duplicated here, although numerically there did appear to be slight increases. This could be due to differences in the procedure (60 min instead of 30 min sac time) as well as examining zif268 protein rather than zif268 mRNA. It should also be noted that their experiment examined immediate early gene expression in this paradigm during adolescence, whereas our experiment used adult rats. Despite a lack of increased IEG expression, we found that inhibition of the pRSC during the period in which contextual information is acquired affected later memory for the context. This suggests that pRSC activity is necessary for consolidation of the context memory but that context exposure itself is not sufficient to cause changes in IEG expression. This pattern of results might instead suggest that inhibiting neural activity in the pRSC during context exposure instead affects behavior via inhibition of efferent neurons to the dorsal hippocampus (Sugar et al. 2011; Wang et al. 2016), a region critically important for context memory (Matus-Amat et al. 2004).
The current pattern of c-fos results is in line with others (Jenkins et al. 2002) who found that memory testing using a radial arm maze resulted in increased c-fos expression during memory retrieval. Interestingly, this was also the case when testing occurred in a novel context supporting a role for the retrosplenial cortex in integrating multiple aspects of complex memories for successful retrieval as we (Trask et al. 2021b) and others (Todd and Bucci 2015) have also supported.
While we predicted that only aRSC activity would be needed during the training phase, when the context and shock presumably become integrated, inhibition of either the aRSC or pRSC during training impaired memory. Recall that the pRSC was needed for memory retrieval, as optogenetic inhibition of this region impacted freezing behavior during the testing phase. This result was mirrored by elevated zif268 expression in both regions following the phase where the context and shock become associated. Together, these results suggest that the inactivation of the posterior RSC during the training period where the shock becomes integrated with the representation of the context prevents formation of the context-shock association by preventing the successful retrieval of the context. Based on our prior work describing separable roles for the anterior and posterior RSC regions in encoding event-related and context-related information separately (Trask et al. 2021a), the likely explanation for these complementary results is that while inhibition of the aRSC during the shock presentations results in reduced freezing by preventing encoding of the shock-related information, inhibition of the pRSC produces the same result by preventing successful retrieval of the context representation learned the previous day. Failure to successfully encoded either the pRSC-mediated context representation or the aRSC-mediated shock representation results in the same reduction in behavioral performance to the context.
Related experiments have tested the role of the dorsal hippocampus, which is widely known to support contextual memory, in this paradigm. For example, Young et al. (1994) found that pre-training lesions of this region impaired fear conditioning to the context and this could be alleviated by pre-exposure to the context 28 days before. This finding was replicated using the context pre-exposure facilitation effect in that permanent DH lesions prior to pre-exposure reduce the CPFE (Rudy et al. 2002). Interestingly, zif268 mRNA is elevated in the DH following pre-exposure, but not footshock, in this paradigm (Heroux et al., 2018). Interfering with memory consolidation in the DH following pre-exposure eliminates the CPFE (Barrientos et al. 2002a, 2002b). Together with the present results, this suggests that the role of the dorsal hippocampus in the context pre-exposure facilitation effect is to support a full representation of the context while the role of the RSC might be more central in integrating distinct aspects of memory. In line with this view, both the aRSC and pRSC showed elevated zif268 and c-fos activity during the training phase in which information about the context and shock become integrated. Further, optogenetic inhibition of either region during this phase impaired memory formation suggesting a failure to associate the context with the shock.
Like the posterior RSC, temporary inactivation of the DH prior to pre-exposure, shock, or testing reduced freezing during the testing phase (Matus-Amat et al. 2004). Mirrored findings were not observed in the aRSC, which is perhaps unsurprising given the more extensive direct connections between the posterior RSC and the DH (Yamawaki et al. 2018). Matus-Amat et al. (2004) similarly suggest that the inactivation at shock prevented a retrieval of the context representation resulting in a failure to link the context and shock. Unlike the DH (Asok et al. 2013), however, both the pRSC and aRSC showed elevated IEG expression following footshock, in line with theories that suggest a role for information integration within the retrosplenial cortex during memory acquisition (Todd and Bucci 2015; Miller et al., 2021; Trask et al. 2021a) and a more specific role for the DH in context memoryonly.
Using a similar, but not identical procedure, Stujenske et al. (2015) demonstrated that when stimulating tissue directly at 5 mW and not through a skull window, light propagation and cellular changes are observed roughly 1 mm beyond the light source. Given this, 5 mW light stimulation through the thinned skull window likely does not extend to more ventral regions of the RSC, these effects are likely due to perturbation of activity primarily in the more dorsal dysgranular layer of the RSC, in line with other work showing a role for the dysgranular RSC in learning and memory (Hindley et al. 2014). Our interpretation of this remains cautious, however, as the depth of light penetration through the tissue was not explicitly measured in the present set of experiments. Future work will need to examine the more distinct contributions of granular and dysgranular RSC layers throughout the rostro-caudal axis of the region.
A related point is that while the present experiments were specifically designed to examine how the anterior and posterior subregions of the RSC contributed to learning and memory for context, both the IEG analysis and the optogenetic manipulation employed here examined the RSC in bulk without subdividing the analyses based on cortical layer or cell type. Given this broad analysis, future work should focus on how contextual fear memory formation and retention affect IEG expression within each cortical layer and how optogenetic manipulation of the distinct cell types within each layer (e.g., pyramidal cells, interneurons, etc.) affect memory for context. However, research examining IEG expression during memory formation using a discrimination task found no differences in c-fos expression between superficial and deep cortical layers (Powell et al. 2017), suggesting that this type of memory formation is not dependent on differential activation of a specific RSC cortical layer.
Together, our results demonstrate differential patterns of involvement in the anterior and posterior retrosplenial cortex in associative memory formation and retrieval. While the anterior portion of the RSC seems to only be necessary during the period where the representation of the environment becomes integrated with the shock, the posterior RSC is needed during each phase of the experiment likely due to its overarching role in contextual processing. Importantly, these results support the hypothesized role of the retrosplenial cortex in memory formation and integration (Todd and Bucci 2015) and demonstrate that activity in both subregions during the time where distinct aspects of the memory become integrated is important successful memory performance.
Contributor Information
Sydney Trask, Department of Psychology, University of Wisconsin-Milwaukee, Milwaukee, WI 53201, USA.
Fred J Helmstetter, Department of Psychology, University of Wisconsin-Milwaukee, Milwaukee, WI 53201, USA.
Funding
National Institutes of Health grants MH069558 (to F.J.H.) and F32MH120938 (to S.T.).
Notes
We thank Dr Nicole Ferrara for comments on the manuscript. Conflict of Interest: The authors declare no competing interest.
References
- Asok A, Schreiber WB, Jablonski SA, Rosen JB, Stanton ME. 2013. Egr-1 increases in the prefrontal cortex following training in the context preexposure facilitation effect (CPFE) paradigm. Neurobiol Learn Mem. 106:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. 2002a. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 134:291–298. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, O'Reilly RC, Rudy JW. 2002b. Memory for context is impaired by injecting anisomycin into dorsal hippocampus following context exploration. Behav Brain Res. 134:299–306. [DOI] [PubMed] [Google Scholar]
- Cain DP, Humpartzoomian R, Boon F. 2006. Retrosplenial cortex lesions impair water maze strategies learning or spatial place learning depending on prior experience of the rat. Behav Brain Res. 170:316–325. [DOI] [PubMed] [Google Scholar]
- Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, Mayford M. 2014. Direct reactivation of a coherent neocortical memory of context. Neuron. 84:432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury N, Quinn JJ, Fanselow MS. 2005. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behav Neurosci. 119:1396. [DOI] [PubMed] [Google Scholar]
- Cullen PK, Ferrara NC, Pullins SE, Helmstetter FJ. 2017. Context memory formation requires activity-dependent protein degradation in the hippocampus. Learn Mem. 24:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landeta AB, Pereyra M, Medina JH, Katche C. 2020. Anterior retrosplenial cortex is required for long-term object recognition memory. Sci Rep. 10:4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. 1986. Associative vs topographical accounts of the immediate shock-freezing deficit in rats: implications for the response selection rules governing species-specific defensive reactions. Learn Motiv. 17:16–39. [Google Scholar]
- Ferrara NC, Trask S, Pullins SE, Helmstetter FJ. 2019. The dorsal hippocampus mediates synaptic destabilization and memory lability in the amygdala in the absence of contextual novelty. Neurobiol Learn Mem. 166:107089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. 2005. Discovering statistics using SPSS. Thousand Oaks (CA): Sage. [Google Scholar]
- Harker KT, Whishaw IQ. 2002. Impaired spatial performance in rats with retrosplenial lesions: Importance of the spatial problem and the rat strain in identifying lesion effects in a swimming pool. J Neurosci. 22:1155–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindley EL, Nelson AJ, Aggleton JP, Vann SD. 2014. Dysgranular retrosplenial cortex lesions in rats disrupt cross-modal object recognition. Learn Mem. 21:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins TA, Dias R, Amin E, Brown MW, Aggleton JP. 2002. Fos imaging reveals that lesions of the anterior thalamic nuclei produce widespread limbic hypoactivity in rats. J Neurosci. 22:5230–5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. 2008a. Neurotoxic lesions of retrosplenial cortex disrupt signaled and unsignaled contextual fear conditioning. Behav Neurosci. 122:1070–1077. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. 2008b. Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behav Neurosci. 122:89–97. [DOI] [PubMed] [Google Scholar]
- Keiser AA, Turnbull LM, Darian MA, Feldman DE, Song I, Tronson NC. 2017. Sex differences in context fear generalization and recruitment of hippocampus and amygdala during retrieval. Neuropsychopharmacology. 42:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lee JL, Gilmartin MR, Helmstetter FJ. 2014. Extinguishing trace fear engages the retrosplenial cortex rather than the amygdala. Neurobiol Learn Mem. 113:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lee JL, Helmstetter FJ. 2015. The retrosplenial cortex is involved in the formation of memory for context and trace fear conditioning. Neurobiol Learn Mem. 123:110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landeira-Fernandez J, DeCola JP, Kim JJ, Fanselow MS. 2006. Immediate shock deficit in fear conditioning: effects of shock manipulations. Behav Neurosci. 120:873. [DOI] [PubMed] [Google Scholar]
- Lee JL, Di Ciano P, Thomas KL, Everitt BJ. 2005. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 47:795–801. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. 2004. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 24:2431–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AM, Mau W, Smith DM. 2019. Retrosplenial cortical representations of space and future goal locations develop with learning. Curr Biol. 29:2083–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AMP, Serrichio AC, Smith DM. 2021. Dual-factor representation of the environmental context in the retrosplenial cortex. Cereb Cortex. 31:2720–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neave N, Lloyd S, Sahgal A, Aggleton JP. 1994. Lack of effect of lesions in the anterior cingulate cortex and retrosplenial cortex on certain tests of spatial memory in the rat. Behav Brain Res. 65:89–101. [DOI] [PubMed] [Google Scholar]
- Powell AL, Vann SD, Olarte-Sánchez CM, Kinnavane L, Davies M, Amin E, Nelson AJ. 2017. The retrosplenial cortex and object recency memory in the rat. Eur J Neurosci. 45:1451–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajbhandari AK, Zhu R, Adling C, Fanselow MS, Waschek JA. 2016. Graded fear generalization enhances the level of cfos-positive neurons specifically in the basolateral amygdala. J Neurosci Res. 94:1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Poorman CE, Marder TJ, Bucci DJ. 2012. Identification of functional circuitry between retrosplenial and postrhinal cortices during fear conditioning. J Neurosci. 32:12076–12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O'Reilly RC. 2002. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci. 116:530–538. [DOI] [PubMed] [Google Scholar]
- Stujenske JM, Spellman T, Gordon JA. 2015. Modeling the spatiotemporal dynamics of light and heat propagation for in vivo optogenetics. Cell Rep. 12:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar J, Witter MP, Strien N, Cappaert N. 2011. The retrosplenial cortex: intrinsic connectivity and connections with the (para) hippocampal region in the rat. An interactive connectome. Front Neuroinform. 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP, Bucci DJ. 2015. Retrosplenial cortex and long-term memory: molecules to behavior. Neural Plast. 2015:414173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP, DeAngeli NE, Jiang MY, Bucci DJ. 2017. Retrograde amnesia of contextual fear conditioning: Evidence for retrosplenial cortex involvement in configural processing. Behav Neurosci. 131:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask S, Pullins SE, Ferrara NC, Helmstetter FJ. 2021a. The anterior retrosplenial cortex encodes event-related information and the posterior retrosplenial cortex encodes context-related information during memory formation. Neuropsychopharmacology. 46:1386–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask S, Ferrara NC, Grisales K, Helmstetter FJ. 2021b. Optogenetic inhibition of either the anterior or posterior retrosplenial cortex disrupts retrieval of a trace, but not delay, fear memory. Neurobiol Learn Mem. 185:107530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. 2002. Extensive cytotoxic lesions of the rat retrosplenial cortex reveal consistent deficits on tasks that tax allocentric spatial memory. Behav Neurosci. 116:85–94. [PubMed] [Google Scholar]
- Vann SD, Wilton LK, Muir JL, Aggleton JP. 2003. Testing the importance of the caudal retrosplenial cortex for spatial memory in rats. Behav Brain Res. 140:107–118. [DOI] [PubMed] [Google Scholar]
- Wang J, Nie B, Duan S, Zhu H, Liu H, Shan B. 2016. Functionally brain network connected to the retrosplenial cortex of rats revealed by 7T fMRI. PLoS ONE. 11:e0146535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki N, Corcoran KA, Guedea AL, Shepherd GM, Radulovic J. 2018. Differential contributions of glutamatergic hippocampal→ retrosplenial cortical projections to the formation and persistence of context memories. Cereb Cortex. 29:2728–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SL, Bohenek DL, Fanselow MS. 1994. NMDA processes mediate anterograde amnesia of contextual fear conditioning induced by hippocampal damage: Immunization against amnesia by context preexposure. Behav Neurosci. 108:19. [DOI] [PubMed] [Google Scholar]


