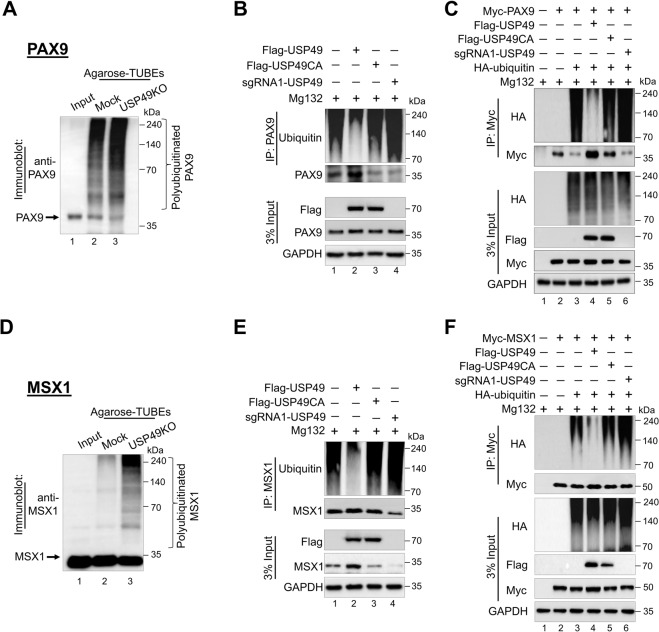
Fig. 4. USP49 deubiquitinates PAX9 and MSX1.
A TUBEs assay for the ubiquitination of PAX9 was conducted in mock-hDPSCs and USP49 KO-hDPSCs using TUBEs-agarose beads. Cell lysates were immunoprecipitated with TUBEs antibodies, followed by immunoblotting with the specific anti-PAX9 antibody. Lane 1, input; Lane 2, mock-hDPSCs; and Lane 3, USP49 KO-hDPSCs. The presented immunoblots are representative of two independent experiments (n = 2). B The ubiquitination and deubiquitination of endogenous PAX9 were analyzed by transfecting hDPSCs with Flag-USP49, Flag-USP49CA, and sgRNA targeting USP49 followed by immunoprecipitation with an anti-PAX9 antibody and immunoblotting with an anti-ubiquitin antibody. The cells were then treated with MG132 for 6 h prior to harvest for all these experiments. The presented immunoblots are representative of two independent experiments (n = 2). C HEK293 cells were transfected with Myc-PAX9, HA-ubiquitin, Flag-USP49, Flag-USP49CA, and sgRNA targeting USP49. The deubiquitination of PAX9 was confirmed by co-immunoprecipitation with the anti-Myc antibody and immunoblotting with the anti-HA antibody. The presented immunoblots are representative of two independent experiments (n = 2). D The TUBEs assay for the ubiquitination of MSX1 proteins was conducted in mock-hDPSCs and USP49 KO-hDPSCs using TUBEs-agarose beads. Cell lysates were immunoprecipitated with the TUBEs antibody followed by immunoblotting with the specific anti-MSX1 antibody. Lane 1, input; Lane 2, mock-hDPSCs; Lane 3, USP49 KO-hDPSCs. The presented immunoblots are representative of two independent experiments (n = 2). E The ubiquitination and deubiquitination of endogenous MSX1 were analyzed by transfecting hDPSCs with Flag-USP49, Flag-USP49CA, and sgRNA targeting USP49, followed by immunoprecipitation with an anti-MSX1 antibody and immunoblotting with an anti-ubiquitin antibody. The cells were then treated with MG132 for 6 h prior to harvest for all experiments. The presented immunoblots are representative of two independent experiments (n = 2). F HEK293 cells were transfected with Myc-MSX1 and HA-ubiquitin, Flag-USP49, Flag-USP49CA, and sgRNA targeting USP49. The deubiquitination of MSX1 was confirmed by co-immunoprecipitation with an anti-Myc antibody and immunoblotting with an anti-HA antibody. The presented immunoblots are representative of two independent experiments (n = 2).