Abstract
Visna-maedi is a multisystemic and progressive inflammatory disease caused by a non-oncogenic retrovirus (Visna-maedi virus, VMV). An outbreak of visna-maedi occurred in Southern Brazil in sheep with clinical signs of blindness and stumbling gait. At post-mortem examination, all animals had similar lesions, including heavy non-collapsed lungs and multifocal yellow areas in the cerebral white matter, affecting mainly the periventricular region. These lesions corresponded histologically to lymphocytic interstitial pneumonia and histiocytic periventricular encephalitis surrounding areas of necrosis, in addition to significant demyelination in the brain. Serology was performed in all the sheep from the flock and 14% were seropositive for VMV. The presence of VMV was confirmed through PCR and partial sequencing of the 5′LTR. Sequencing demonstrated that the virus had 89.7 to 90.0% of nucleotide identity with VMV strains reported in the USA. This is the first description of clinical disease related to VMV in Brazil leading to economic losses. This study calls for the need to implement control measures to prevent the spread of small ruminant lentiviruses in Brazil.
Keywords: Visna-maedi, Ovine, Lentivirus, Meningoencephalomyelitis, Interstitial pneumonia
Introduction
Small ruminant lentiviruses (SRLV) are non-oncogenic single-stranded RNA retroviruses that infect sheep and goats, leading to diseases known as visna-maedi (VM; also known as ovine progressive pneumonia) and caprine arthritis and encephalitis (CAE) [8]. Both diseases are multisystemic, progressive, and degenerative, with the establishment of persistent viral infection via viral genomic integration into the host’s genome [6, 14, 31]. The etiological agents of VM and CAE are Visna-maedi virus (VMV) and caprine arthritis encephalitis virus (family Retroviridae, genus Lentivirus), respectively. These viruses present high mutation and recombination potential, and five main genotypes (A–E), with more than 28 subgroups characterized worldwide. The phylogenetic diversity implies high genetic and antigenic diversity, which interferes with serologic and molecular diagnosis [24]. While Europe presents the highest individual seroprevalence (40.9%), South and Central America show the lowest individual SRLV prevalence (1.7%) [22]. In Brazil, there are reports of VMV seroprevalence in many regions, as well as reports of phylogenetic analysis of sheep isolates from some of the Southern States (Rio Grande do Sul and Paraná) [13, 25]; however, only subclinical disease was reported [13].
VMV is immunopathogenic, leading to the infiltration of tissues by mononuclear cells, including subsets of lymphocytes, macrophages, and plasma cells. This inflammatory process ultimately causes the destruction of tissue architecture and interferes in tissue function [8]. The four major tissues affected by VMV are the lungs, brain/spinal cord, articular cartilage, and mammary gland [8, 14]. The pulmonary disease (maedi) is the most commonly observed in sheep and is characterized as lymphocytic interstitial pneumonia [6, 8, 14], while the neurologic syndrome (visna) is consistent with meningoencephalitis. Both chronic progressive arthritis and non-suppurative mastitis are less frequently caused by SRLV in the ovine species [8]. This case report aims to describe the pathological and molecular features of the first outbreak of VMV-associated clinical disease in a flock of sheep in Brazil.
Materials and methods
We investigated an outbreak of neurologic and respiratory disease in a flock of 96 sheep located in the metropolitan region of Porto Alegre, Brazil. Clinical and epidemiological data from the outbreak were provided by the farm owner and by the veterinarian. Blood samples were collected from all sheep and submitted for serological testing, using a modification of the method described by Castro [12]. Both labeled avidin-biotin ELISAs (VMV, CAEV, and p28 Rec) and AGID were performed. The indirect ELISAs used VMV and CAEV obtained through cell culture, concentrated by polyethylene glycol, and treated with Triton X 100, and a recombinant p28 antigen (ELISA p28 Rec — produced in Escherichia coli). The AGID assay was performed using 100-mm Petri dishes containing 1.1% agar noble (Difco, USA) in borate buffer (0.05 mol/L NaOH and 0.016 mol/L H3BO3). The plates were incubated at room temperature in a humidified chamber for 48 h. One or two lines showing identity with the positive standard serum between a test serum and the antigen were interpreted as a positive result.
Three sheep (ewe 1, 2, and 3) with worsening neurologic signs were euthanized with an intravenous overdose of sodium thiopental. Immediately after euthanasia, a complete post-mortem examination and sample collection were performed on all sheep, and gross lesions were recorded. Formalin-fixed samples including the brain, lungs, mammary gland, synovial capsule, and other main organs were routinely processed, sectioned (3-μm thick), and stained with hematoxylin and eosin (H&E) for histopathological examination. Representative sections of the brain and the spinal cord, in addition to sections of the brain from a sheep without neurological signs (control), were also stained following the Luxol Fast Blue method. In addition, immunohistochemical (IHC) analyses were performed on representative serial sections of the lung, brain, and spinal cord of all three sheep using the peroxidase-labeled universal polymer method (MACH 4, Universal HRP-Polymer, Biocare Medical, Pacheco, CA, USA) for the following antibodies: CD3 (ready-to-use; Dako) and CD79α (1:100; Dako). The chromogen used for visualization was 3,3-diaminobenzidine (DAB, Sigma, St Louis, MO, USA). Iba-1 immunostaining was performed at Louisiana Animal Disease Diagnostic Laboratory using the Leica BOND-MAX automated platform and the Polymer Refine Detection kit (Leica Biosystems, Buffalo Grove, IL, USA). Briefly, sections were automatically deparaffinized and subjected to heat-induced antigen retrieval using the ER1 solution (Leica Biosystems) for 20 min at 100 °C. Sections were incubated with anti-Iba-1 antibody (1:2,000, Wako Chemicals, Richmond, VA, USA) for 30 min followed by a polymer-labeled goat anti-rabbit conjugated with horseradish peroxidase (8 min) and DAB as the chromogen (10 min). Positive controls consisted of feline pulmonary Langerhans cell histiocytosis (Iba-1) and canine and sheep lymph nodes (CD3 and CD79α). For the negative reagent control, a universal negative control for mouse and rabbit antibodies (Biocare) was used instead of the primary antibody. Immunohistochemistry sections were counterstained with Harris hematoxylin.
During post-mortem examination, we collected a swab of the abscesses of the mammary gland of ewe 2 and submitted it for bacteriological culture. Samples were cultured aerobically at 37 °C for 72 h on Blood Agar (5% sheep blood Mueller Hinton, Kasvi®, Brazil) and MacConkey Agar plates (Kasvi ®, Brazil). Isolated colonies were identified through morphology and conventional biochemical tests.
Fresh samples of the brain and lungs of the euthanized sheep were utilized for nested PCR analysis as described by Ryan [28]. Briefly, the brain and lungs were macerated and diluted to 20% (w/v) in phosphate-buffered saline (PBS) (pH 7.2), centrifuged at low speed (1800 ×g for 30 min), filtered through a 0.45-μm filter for removal of small debris, and stored at −80 °C for subsequent analysis. The macerated brain and lungs of each animal were pooled, proviral DNA was isolated using a standard phenol-chloroform protocol [29], and the nested PCR was performed using a protocol that amplifies a 460-bp fragment from the proviral 5′ LTR resulting in a 400-bp sequence after deleting the primer sequences and sequences with a low quality close to the primers [28]. The resultant PCR products were purified using the PureLink™ Quick PCR Purification Kit (Invitrogen, Carlsbad, CA, USA). Both DNA strands were sequenced with an ABI PRISM 3100 Genetic Analyzer using a BigDye Terminator v.3.1 cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA). Overlapping fragments were aligned and assembled using Geneious software [16]. A total of 11 SRLV 5′ LTR sequences comprising genotypes A, B, and C were retrieved from GenBank and aligned using the MUSCLE algorithm available in the MEGA 6 software [32]. The phylogenetic tree was constructed using neighbor-joining inference and Kimura-2 substitution model in 1000 bootstrap replicates using the MEGA 6 software [32].
Results
Epidemiological and clinical findings
The outbreak occurred in January 2018 in a flock of sheep in Salvador do Sul, Rio Grande do Sul, Southern Brazil (29° 26′ 12″ S, 51° 29′ 27″ W). The sheep were raised in a semi-intensive grazing system for lamb production. The sheep grazed in a Tifton pasture during daylight hours and were gathered indoors at night. For reproduction, artificial insemination was performed using commercially available semen. Sheep from different origins were purchased and introduced into the flock without testing for SRLV, approximately 2.5 years before the outbreak occurred. The flock consisted of 96 female Texel sheep, ranging from 5 months to 7 years old. Of the 96 sheep, 55 were adults, and 41 were lambs. Lambs were raised by their dams until 2–3 months of age. Fourteen adult sheep (average age of 3 years) were serologically positive for VMV. The morbidity rate was 14.5%. No lambs were seropositive. All seropositive sheep had mild respiratory signs characterized by cough and dyspnea and were eventually separated from the flock and later eliminated. Three seropositive ewes, which were 4, 7, and 4 years old, were blind, with stumbling gait, head tilt, anorexia, and progressive weight loss of 2-month duration. Due to poor prognosis, these three sheep, designated ewes 1, 2, and 3, respectively, were humanely euthanized.
Pathological findings
At gross examination, the three euthanized ewes were in poor body condition. The cerebrum of one of the sheep (ewe 1) had multifocal to coalescing, irregular, yellow to brown, soft areas delimiting the periventricular region (Fig. 1a). Similar gross alterations were noted in the other two sheep but of less severity. In all animals examined, the lungs were heavy and non-collapsed, with rib impressions. The affected lungs were pale gray to pink, with a diffusely rubbery texture (Fig. 2a) and, on the cut surface, multiple white and firm foci, measuring 0.1 to 0.5 cm in diameter were observed, mainly around bronchi (Fig. 2a inset). The mammary gland of ewe 2 had multifocal abscesses with caseous exudate. The mammary glands of the other two ewes were mildly hardened. No significant lesions were observed in other organs, including multiple joints (stifle, carpi, and tarsi).
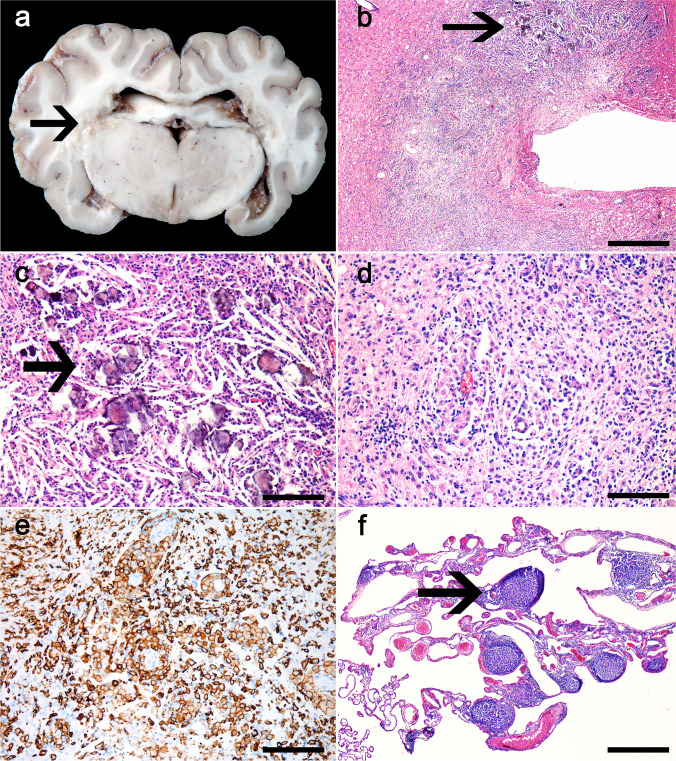
Fig. 1.
Visna-maedi virus-infected sheep. a Ewe 1: Grossly, the cerebrum has multifocal to coalescing, irregular, yellow to brown, soft, periventricular areas (arrow). b–f Histologic findings. b Periventricular areas of the brain with necrosis, severe inflammatory infiltrate, cholesterol clefts, and deposition of mineral (arrow). H&E, 600 μm. c Higher magnification of the periventricular areas with deposition of mineral material (arrow), cholesterol clefts, and mononuclear infiltrate. H&E, 100 μm. d Marked infiltrate of macrophages and a smaller number of lymphocytes. H&E, 100 μm. e Numerous strongly immunolabeled macrophages infiltrate the white matter. IHC anti-Iba1, 100 μm. f The choroid plexus is expanded by multifocal infiltration of lymphocytes in the form of follicles-like structures (arrow). H&E, 600 μm
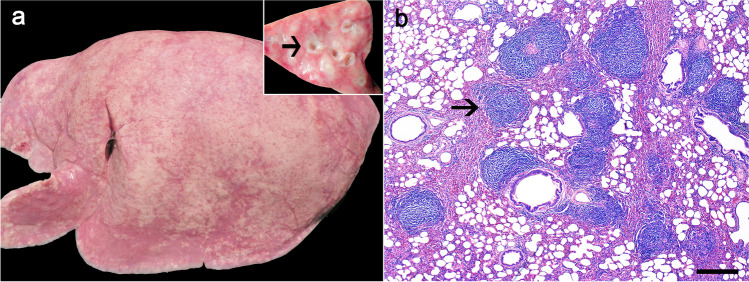
Fig. 2.
Visna-maedi virus-infected sheep. a Grossly, the lungs of all examined sheep were pale gray to pink and non-collapsed, with rib impressions and a diffusely rubbery texture. Inset. On the cut surface, multifocal white areas were present throughout the pulmonary parenchyma, mainly surrounding bronchi (arrow). b Histologic findings. The lungs had interstitial pneumonia with distinct large lymphoid follicle-like structures (arrow) around bronchi, bronchioles, and vessels. H&E, 600 μm
Microscopically, all cases exhibited similar changes. In the cerebrum, the periventricular yellowish areas observed grossly consisted of extensive necrosis (Fig. 1b–d), mainly on the white matter, with numerous macrophages, strongly Iba-1+ (Fig. 1e), and fewer lymphocytes, predominantly CD3+ (T) cells and less frequently CD79α+ (B) cells, cholesterol clefts, hemorrhage, and mineral deposits (necrotizing leukoencephalitis). The remaining white matter had moderate rarefaction, also demonstrated by the markedly reduced myelin staining on the Luxol Fast Blue-stained sections, and multifocal moderate perivascular cuffing composed of macrophages and fewer lymphocytes. The choroid plexuses of the lateral ventricles were multifocally expanded by abundant fibrin and a moderate infiltrate mainly composed of lymphocytic aggregates forming lymphoid follicle-like structures, and a small number of macrophages (choroid plexitis) (Fig. 1f). In the brainstem, the leptomeninges were moderately expanded by similar areas of perivascular infiltrate. The lesions in the spinal cord, mainly in the lumbar and thoracic segments, consisted of multifocal areas of necrosis with interstitial and perivascular macrophages and fewer lymphocytes and plasma cells (necrotizing myelitis). In the Luxol Fast Blue-stained sections of the spinal cord, there is moderately reduced myelin staining in the white matter of the cervical and lumbar spinal cord segments and markedly reduced staining in the white matter of the thoracic spinal cord. The lungs had interstitial pneumonia with multiple lymphocytic aggregates forming lymphoid follicle-like structures surrounding bronchi, bronchioles, and vessels (Fig. 2b). The alveolar septa were moderately thickened by smooth muscle hypertrophy, type II pneumocyte hyperplasia, and moderate infiltration of lymphocytes and few macrophages, strongly CD3+ (T) lymphocytes, and low to moderate numbers of Iba-1+ cells. The mammary gland of all ewes had multifocal interstitial lymphocytic aggregates and, in ewe 2, we also observed multifocal areas of necrosis with degenerate neutrophils surrounded by connective tissue proliferation (abscesses). Culture of the swab of the mammary gland abscesses yielded mixed growth of Trueperella pyogenes and Staphylococcus sp. The synovial membrane was also evaluated histologically; however, no lesions were observed.
Molecular analysis
The nested PCR for VMV was positive on the tissue pool (brain and lung) of all three analyzed sheep. Sequencing of the 460-bp fragment amplified from the proviral 5′ LTR resulted in a 400-bp sequence that showed 90.0% and 89.7% of identity with SRLV strains detected in sheep in the USA (GenBank accession numbers AY101611.1 and HM053008.1). The partial 5′LTR nucleotide phylogenetic tree (Fig. 3) represented the three different clades of the SRLV genotypes A, B, and C. The genotype A clade also included the subtypes A1, A2, and A3. Phylogenetic analysis of the sequences generated in the present outbreak grouped with the genotype A, subtype A2 (GenBank accession number MZ126603.1) and were determined to be closely related to SRLVs detected in sheep in the USA (GenBank accession numbers AY101611.1, AF012296, L19199, and U39826).
Fig. 3.
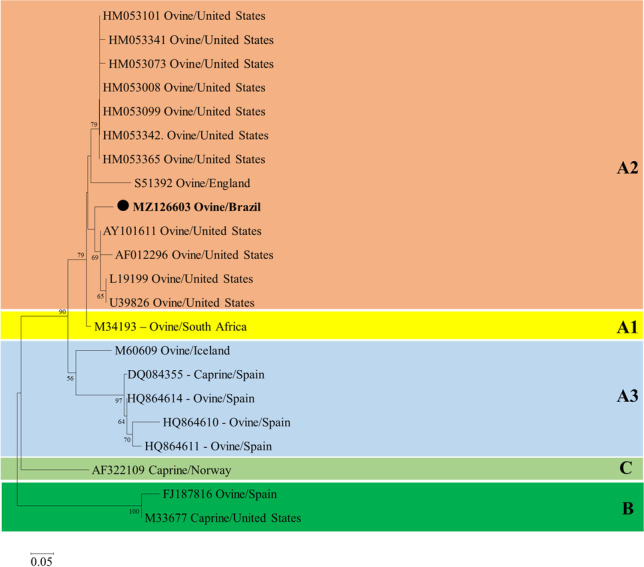
Partial 5′LTR nucleotide phylogenetic tree of small ruminant lentiviruses. The nucleotide sequences were analyzed by the neighbor-joining method with the Kimura-2 model and 1000 bootstrap replicates. The percentage of replicate trees in which the sequences clustered together are shown next to the branches if >50%. GenBank accession numbers are represented, and the sequence detected in the present study has been highlighted with ● (GenBank accession number MZ126603.1)
Discussion
Sheep infected with VMV develop a chronic wasting syndrome characterized by persistent infection in several organs, including lungs, central nervous system, mammary gland, and joints [6, 31]. The respiratory form (maedi) is characterized by interstitial pneumonia and is the most common form of the disease. Clinical signs consist of progressive weight loss and dyspnea [6, 14]. The neurologic form of the disease (visna) is sporadic and was first described in Iceland as non-suppurative demyelinating encephalitis clinically characterized by a chronic progressive paralytic disease of adult sheep [31]. Studies demonstrated the low seroprevalence of VMV on the Brazilian sheep flock; however, clinical disease was never reported. In the current outbreak, although the neurological signs were the main reason for the investigation, interstitial pneumonia was still prominent. The lesions in the spinal cord are thought to have developed by extension from the brain; however, some sheep have demyelinating mononuclear inflammatory lesions limited to the spinal cord [6, 7]. In the sheep in the current outbreak, the spinal cord lesions were most severe in the thoracic segments as also described in the literature [5, 34].
Clinical signs of visna are not pathognomonic; therefore, pathological and ancillary laboratory testing is necessary to differentiate this condition from those caused by other pathogens. Differential diagnoses for neurological disease in sheep include listeriosis, Border disease, rabies, louping-ill, polioencephalomalacia, coenurosis, and scrapie [15, 26, 27]. Of these diseases, rabies is common in ruminants in Rio Grande do Sul, although it is rarely reported in sheep [4, 27]. Rabies in sheep is a paralytic disease, with stumbling gait and hindlimb paresis/paralysis [27]. This clinical feature of rabies can be mistaken with the neurologic form of SRLV, as described in our cases, in which the sheep demonstrated similar neurologic signs. Nonetheless, the time lapse between the onset of clinical signs and death significantly differs between the two diseases. Rabies has acute clinical progression (approximately 5 days); visna is instead a chronic and progressive disease that can last for weeks or months [6, 27].
VMV infection of sheep in Brazil was previously determined based on serological studies (Table 1). Even if serology demonstrates that the prevalence of visna-maedi is low or absent, epidemiological surveillance is necessary to ensure an adequate health status of the flock, since the disease can lead to severe losses in sheep production [17, 18]. SRLV-positive animals can be determined with multiple serological and molecular tests, including ELISA and PCR. In the current outbreak, we performed ELISA to obtain the seroprevalence and identify positive sheep in order to eliminate the virus. PCR was performed on lung and brain collected at necropsy to confirm and characterize the virus. In our study, the amplified segment of the genome (5′LTR) had 90.0% and 89.7% of identity with VMV strains reported in the USA. Our analyses, therefore, suggest that the present isolate may be related to the North American viral strains. It is also significantly different from the other VMV strains previously identified in Southern Brazil [25]. Small ruminant lentiviruses have been classified into five genetic groups (A–E), which differ from each other in 25–37% of their nucleotide sequences. Genotypes A, B, and E, originally described in sheep (A) or goats (B and E), are distributed into different subtypes (A1–A15, B1–B3, E1–E2) [24]. The SRLV detected in the present outbreak was classified phylogenetically as subtype A2, which is commonly associated with infections in sheep [30]. The time and tissue in which lesions develop depend on the strain of the virus and the genetic background of the host [8]. One study determined that the neurovirulence of VMV can be linked to duplication of CAAAT sequences in the viral long terminal repeat (LTR) [23]. A specific deletion located inside LTRs could also be associated with the low pathogenicity of some strains of SRLV [2]. This difference in the strains may be the reason why in Brazil no clinical disease has been reported despite several serologic studies demonstrating the presence of SRLV in the sheep population.
Table 1.
Seroprevalence of SRLV in sheep in the Brazilian territory
| Region | State | Number of sheep tested | Prevalence (%) | Reference |
|---|---|---|---|---|
| North | ||||
| Amazonas | 122 | 8.2 | [36] | |
| Tocantins | 369 | 1.62 | [19] | |
| Northeast | ||||
| Bahia (Juazeiro) | 200 | 0.3 | [18] | |
| Ceará | 1.001 | 0 | [1] | |
| Paraíba | 459 | 0 | [1] | |
| Pernambuco | 383 | 0.26 | [20] | |
| Rio Grande do Norte | 931 | 0 | [1] | |
| Sergipe | 941/931 | 0.11/0 | [1, 21] | |
| Maranhão | 1,495 | 0.5; 0.7; 1.0 | [33] | |
| Southeast | ||||
| São Paulo | 444 | 2.7 | [17] | |
| Espírito Santo | 150 | 7.33 | [3] | |
| South | ||||
| Rio Grande do Sul | 267 | 10.48 | [13] |
The spread of the disease is related to uncontrolled movement of animals without previous testing, as demonstrated for instance in Costa Rica [35]. This also occurred with the flock of this outbreak, in which sheep from different regions were introduced without any type of testing for SRLV; therefore, our hypothesis is that an asymptomatic animal was responsible for introducing the agent to the affected flock. Several management practices are useful to control the spread of VMV, such as separation and elimination of positive animals, testing of genetic material (e.g., semen), and quarantine and testing of imported animals [10]. In this outbreak, the positive sheep in the affected flock were identified and eliminated. No disease recurrence was reported. As for other diseases, such as scrapie and Border disease, active surveillance for SRLV can be performed on third eyelid biopsy [11]; however, this practice has yet to be adopted in Brazil. No effective vaccines are currently available against SRLV [8]; therefore, testing and removal of positive animals are essential for disease control. Another recommendation for disease control is the removal of the lambs, as soon as they are born, from the ewes, to prevent the infection through milk and colostrum ingestion [9].
In conclusion, this outbreak illustrates the occurrence of clinical disease associated with VMV, especially the neurologic form, in a flock of sheep in Southern Brazil. Performing routine serologic testing in Brazilian flocks for VMV is essential to determine the need for control measures that will prevent the spread of the disease.
Acknowledgements
The authors gratefully acknowledge the staff members of the Histology and Immunohistochemistry sections at the Louisiana Animal Disease Diagnostic Laboratory (LADDL), School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA, for their assistance with routine and special stained slide preparation. The authors also gratefully acknowledge the members of the Setor de Patologia Veterinária from the Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil, for their support. The authors also gratefully acknowledge Dr. Roberto Castro and Biovetech for performing the serology tests.
Author contribution
Bianca Santana de Cecco, Luan Cleber Henker, Marina Paula Lorenzett, Franciéli Adriane Molossi, Claiton Ismael Schwertz, Saulo Petinatti Pavarini, David Driemeier, and Luciana Sonne received and diagnosed the cases. Bianca Santana de Cecco wrote the manuscript. Luan Cleber Henker, Marina Paula Lorenzett, Franciéli Adriane Molossi, Saulo Petinatti Pavarini, David Driemeier, and Luciana Sonne contributed also to the review and editing of the manuscript. Matheus Nunes Weber, Letícia Baumbach, and Cláudio Wageck Canal performed virology tests. Mariano Carossino, Udeni Balasuryia, and Ingeborg Maria Langohr contributed with IHC techniques, review, and editing of the manuscript.
Funding
Financial support was supplied by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)- Finance Code 001, Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS), and Pró-reitoria de Pesquisa da Universidade Federal do Rio Grande do Sul (Propesq/UFRGS). This study was also partially supported by Louisiana State University, School of Veterinary Medicine start-up fund (PG 002165) to Dr. Udeni B. R. Balasuriya.
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval
All cases described herein occurred spontaneously, with no experimentation, inoculation, or treatment of live animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Consent to participate
Not applicable.
Consent for publication
All authors read and approved the manuscript.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alves SM, Teixeira MFS, Pinheiro RR, Alves FSF, Lima AMC, Farias DA, Santos VWS, Azevedo DAA, Martins GR, Aguiar TDAF. Seroepidemiological study of maedi-visna in sheep in Ceará, Rio Grande do Norte, Paraíba, and Sergipe States. Semin Cienc Agrar. 2018;39(5):2017–2028. doi: 10.5433/1679-0359.2018v39n5p2017. [DOI] [Google Scholar]
- 2.Angelopoulou K, Brellou GD, Greenland T, Vlemmas I. A novel deletion in the LTR region of a Greek small ruminant lentivirus may be associated with low pathogenicity. Virus Res. 2006;118:178–184. doi: 10.1016/j.virusres.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Barioni G, Pereira LV, Beltrame MAV, Tesoline P, Gumieiro MV. Soroprevalência de maedi-visna em ovinos da raça Santa Inês nos municípios da grande Vitória – ES. Cienc Anim Bras. 2009;1:579–584. [Google Scholar]
- 4.Bassuino DM, Konradt G, Mari C, da Cruz RAS, Boos GS, Gomes DC, Pavarini SP, Driemeier D. Clinical, pathological and immunohistochemical findings of sheep infected with rabies virus. Braz J Vet Med. 2016;38(1):86–90. doi: 10.2430/00000000000000. [DOI] [Google Scholar]
- 5.Benavides J, Fuertes M, García-Pariente C, Ferreras MC, García-Marin JF, Pérez V. Natural cases of visna in sheep with myelitis as the sole lesion in the central nervous system. J Comp Pathol. 2006;134:219–230. doi: 10.1016/j.jcpa.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Benavides J, Gómez N, Gelmetti D, Ferreras MC, García-Pariente C, Fuertes M, Gárcia-Marín JF, Pérez V. Diagnosis of the nervous form of maedi-visna infection with a high frequency in sheep in Castilla y León, Spain. Vet Rec. 2006;158:230–235. doi: 10.1136/vr.158.7.230. [DOI] [PubMed] [Google Scholar]
- 7.Biescas E, Preziuso S, Bulginms Y, DeMartini JC. Ovine lentivirus associated leucomyelitis in naturally infected North American sheep. J Comp Pathol. 2005;132(3):107–116. doi: 10.1016/j.jcpa.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Blacklaws B. Small ruminant lentiviruses: immunopathogenesis of visna-maedi and caprine arthritis and encephalitis virus. Comp Immunol Microbiol Infect Dis. 2012;35:259–269. doi: 10.1016/j.cimid.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Blacklaws B, Berriatua E, Torsteinsdottir S, Watt NJ, de Andres D, Klein D, Harkiss GD. Transmission of small ruminant lentiviruses. Vet Microbiol. 2004;101:199–208. doi: 10.1016/j.vetmic.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Callado AKC, Castro RS, Teixeira MFS. Lentivírus de pequenos ruminantes (CAEV e Maedi-Visna): revisão e perspectivas. Pesqui Vet Bras. 2001;21(3):87–97. doi: 10.1590/S0100-736X2001000300001. [DOI] [Google Scholar]
- 11.Cappuchio MT, Sanna E, Farigu S, Minelli R, Guarda F. Maedi-visna virus detection in ovine third eyelids. J Comp Pathol. 2003;129:37–43. doi: 10.1016/s0021-9975(02)00158-5. [DOI] [PubMed] [Google Scholar]
- 12.Castro RS, Leite RC, Resende M, Gouveia A. A labelled Avidin-Biotin ELISA to detect antibodies to caprine arthritis-encephalitis virus in goat’s sera. Vet Res Commun. 1999;23(8):515–522. doi: 10.1023/A:1006370607924. [DOI] [PubMed] [Google Scholar]
- 13.Dal Pizzol M, Ravazzolo AP, Gonçalves IPD, Hotzel I, Fernandes JCT, Moojen V. MAEDI-VISNA: identificação de ovinos infectados no Rio Grande do Sul, Brasil, 1987-1989. Arq Fac Med Vet UFRGS. 1989;17:65–76. [Google Scholar]
- 14.Dawson M. Pathogenesis of maedi-visna. Vet Rec. 1987;120:451–454. doi: 10.1136/vr.120.19.451. [DOI] [PubMed] [Google Scholar]
- 15.Guedes KMR, Riet-Correa F, Dantas AFM, Simões SVD, Miranda Neto EG, Nobre VMT, Medeiros RMT. Diseases of the central nervous system in goats and sheep of the semiarid. Pesqui Vet Bras. 2007;27(1):29–38. doi: 10.1590/S0100-736X2007000100006. [DOI] [Google Scholar]
- 16.Kearse M, Moir R, Wilson A, Stone-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lombardi AL, Nogueira AHC, Feres FC, Paulo HP, Castro RS, Feitosa FLF, Cadioli FA, Peiró JR, Perri SHV, Lima VFM, Mendes LCN. Soroprevalência de Maedi-Visna em ovinos na região de Araçatuba, SP. Arq Bras Med Vet Zootec. 2009;61(6):1434–1437. doi: 10.1590/S0102-09352009000600025. [DOI] [Google Scholar]
- 18.Martinez MP, Costa JN, Souza TS, Lima CCV, Neto OCN, Pinheiro RR. Prevalência sorológica da maedi-visna em rebanhos ovinos da microrregião de juazeiro. Cienc Anim Bras. 2011;12(2):322–329. doi: 10.5216/cab.v12i2.4454. [DOI] [Google Scholar]
- 19.Mazzinghy CL, Almeida KS, Veschi JLA, Castro RS, Martins NEX, Sousa MG. Frequency of antibodies against ovine Lentivirus in sheep in Colinas do Tocantins, Tocantins state, Brazil. Arq Inst Biol. 2016;83:1–5. doi: 10.1590/1808-1657000542014. [DOI] [Google Scholar]
- 20.Melo EX, Almeida EC, Mendonça KMN, Nascimento SA, Silva JCR, Marvulo MFV, Rizzo H, Castro RS. Seroprevalence small ruminant lentiviruses in the state of Pernambuco slaughterhouses, Brazil. Arq Inst Biol. 2016;83:1–4. doi: 10.1590/1808-1657000462015. [DOI] [Google Scholar]
- 21.Mendonça CED, Barros SLB, Mendonça MAD, Guimarães VAA, Pinheiro RR. Occurrence of antibodies against Maedi-Visna in Santa Inês sheep in the State of Sergipe, Brazil. Arq Inst Biol. 2013;80(3):346–351. doi: 10.1590/S1808-16572013000300013. [DOI] [Google Scholar]
- 22.Miguel R, Arrieta M, Rodríguez-Largo A, Echeverría I, Resendiz R, Pérez E, Ruiz H, Pérez M, Andrés D, Reina R, Blas I, Luján L. Worldwide prevalence of small ruminant lentiviruses in sheep: a systematic review and meta-analysis. Animals. 2021;11:784. doi: 10.3390/ani11030784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oskarsson T, Hreggvidsdóttir HS, Agnarsdóttir G, Matthíasdóttir S, Ogmundsdóttir MH, Jónsson SR, Georgsson G, Ingvarsson S, Andrésson OS, Andrésdóttir V. Duplicated sequence motif in the long terminal repeat of maedi-visna virus extends cell tropism and is associated with neurovirulence. J Virol. 2007;81(8):4052–4057. doi: 10.1128/JVI.02319-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramírez H, Reina R, Amorena B, de Andrés D, Martínez HA. Small ruminant lentiviruses: genetic variability, tropism and diagnosis. Viruses. 2013;5:1175–1207. doi: 10.3390/v5041175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravazzolo AP, Reischak D, Peterhans E, Zanoni R. Phylogenetic analysis of small ruminant lentiviruses from Southern Brazil. Virus Res. 2001;79:117–123. doi: 10.1016/s0168-1702(01)00339-2. [DOI] [PubMed] [Google Scholar]
- 26.Rissi DR, Pierezan F, Kommers GD, Barros CSL. Occurrence of rabies in sheep in Rio Grande do Sul, Brazil. Pesq Vet Bras. 2008;28(10):495–500. doi: 10.1590/S0100-736X2008001000009. [DOI] [Google Scholar]
- 27.Rissi DR, Pierezan F, Oliveira Filho JC, Fighera RA, Irigoyen LF, Kommers GD, Barros CSL. Diseases of sheep from central Rio Grande do Sul State, Brazil: 361 cases. Pesqui Vet Bras. 2010;30(1):21–28. doi: 10.1590/S0100-736X2010000100004. [DOI] [Google Scholar]
- 28.Ryan S, Tiley L, McConnell I, Blacklaws B. Infection of dendritic cells by the Maedi-Visna Lentivirus. J Virol. 2000;74(21):10096–10103. doi: 10.1128/jvi.74.21.10096-10103.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook J, Russel DW. Molecular cloning. A laboratory manual. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 30.Shah CA, Böni J, Huder JB, Vogt HR, Mühllher J, Zanoni R, Miserez R, Lutz H, Schüpbach J. Phylogenetic analysis and reclassification of caprine and ovine lentiviruses based on 104 new isolates: evidence for regular sheep-to-goat transmission and world-wide propagation through livestock trade. Virology. 2004;319:12–26. doi: 10.1016/j.virol.2003.09.047. [DOI] [PubMed] [Google Scholar]
- 31.Sigurdsson B, Pálsson PA, Grímsson H. Visna, a demyelinating transmissible disease of sheep. J Neuropathol Exp Neurol. 1957;16:389–403. doi: 10.1097/00005072-195707000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teixeira WC, Azevedo EO, Nascimento SA, Mavulo MFV, Rizzo H, Silva JCR, Castro RS. Seroprevalence of Maedi-Visna in sheep flocks State of Maranhão, Brazil. R Bras Ci Vet. 2016;23(1-2):42–47. doi: 10.4322/rbcv.2016.028. [DOI] [Google Scholar]
- 34.Vandevelde M, Higgins RJ, Oevermann A. Veterinary neuropathology: essential of theory and practice. West Sussex: Wiley-Blackwell; 2012. [Google Scholar]
- 35.Villagra-Blanco R, Dolz G, Solórzano-Morales A, Alfaro A, Montero-Caballero D, Romero-Zúñiga J. Presence of maedi-visna in Costa Rican sheep flocks. Small Rumin Res. 2015;124:132–136. doi: 10.1016/j.smallrumres.2015.01.010. [DOI] [Google Scholar]
- 36.Vinha KT, Silva TIB. Seropositivity for Maedi-Visna virus in sheep in Porto Acre city – Western Amazon, Brazil. Cienc Anim Bras. 2020;21:e-59173. doi: 10.1590/1809-6891v21e-59173. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.