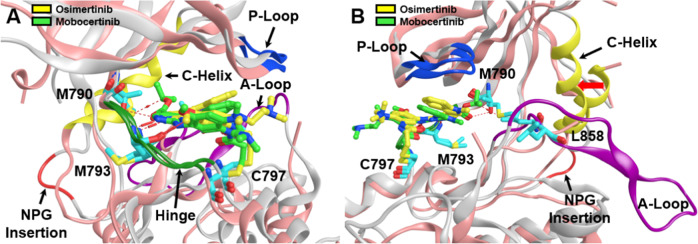
Fig. 3.
Aligned and superposed EGFR ex20ins mutant (light gray) bound to osimertinib (yellow) and EGFR T790M mutant (pink) bound to mobocertinib (light green), showing the active site cleft in two different views (A, B) and highlighting several prominent structural features within the EGFR tyrosine kinase domain: the phosphate-binding P-loop (blue ribbon), the substrate-recruiting activation loop (A-loop, violet ribbon), the C-helix (yellow ribbon), NPG insertion mutation (red ribbon), and the hinge region (green ribbon), as well as key residues (cyan sticks) such as M793 in the “hinge” region, the “gatekeeper” residue mutation T790M, the exon 21 mutation site L858, and C797 responsible for the covalent binding interactions with irreversible TKIs. Red arrow (B) shows inward shift of C-helix due to NPG insertion. Prepared from PDB ID: 7LGS and 7A6K [40, 46]