Abstract
Purpose:
The FLASH effect is characterized by normal tissue sparing without compromising tumor control. Although demonstrated in various preclinical models, safe translation of FLASH-radiotherapy stands to benefit from larger vertebrate animal models. Based on prior results, we designed a randomized phase III trial to investigate the FLASH effect in cat patients with spontaneous tumors. In parallel, the sparing capacity of FLASH-radiotherapy was studied on mini pigs by using large field irradiation.
Experimental Design:
Cats with T1-T2, N0 carcinomas of the nasal planum were randomly assigned to two arms of electron irradiation: arm 1 was the standard of care (SoC) and used 10 × 4.8 Gy (90% isodose); arm 2 used 1 × 30 Gy (90% isodose) FLASH. Mini pigs were irradiated using applicators of increasing size and a single surface dose of 31 Gy FLASH.
Results:
In cats, acute side effects were mild and similar in both arms. The trial was prematurely interrupted due to maxillary bone necrosis, which occurred 9 to 15 months after radiotherapy in 3 of 7 cats treated with FLASH-radiotherapy (43%), as compared with 0 of 9 cats treated with SoC. All cats were tumor-free at 1 year in both arms, with one cat progressing later in each arm. In pigs, no acute toxicity was recorded, but severe late skin necrosis occurred in a volume-dependent manner (7–9 months), which later resolved.
Conclusions:
The reported outcomes point to the caveats of translating single-high-dose FLASH-radiotherapy and emphasizes the need for caution and further investigations.
Translational Relevance.
The FLASH effect has been demonstrated in various preclinical models, and clinical implementation has now begun. However, the safe translation of current preclinical findings to humans requires longer-term follow-up of normal tissue toxicities along with more clinically relevant target volumes. Therefore, to directly address these existing limitations, we designed a randomized phase III trial to investigate the FLASH effect in cat patients with spontaneous tumors with a long-term follow-up. In parallel, the normal tissue sparing capacity of FLASH-radiotherapy was investigated using a large field of irradiation on the skin of mini pigs with a 3.3-year follow-up.
Introduction
In recent years, the ultra-high dose rate delivery of radiotherapy FLASH-radiotherapy has emerged as one of the most promising advancements in the field of radiation oncology. FLASH-radiotherapy was shown to simultaneously delay tumor growth while preventing normal tissue complications (1, 2, 3).
Supported by radiobiological studies, FLASH-radiotherapy is a potentially paradigm-shifting method for delivering doses within a short irradiation time (tenths of a second) at an ultra-high intrapulse dose rate (∼106 Gy/s). FLASH-radiotherapy has been shown to preserve normal tissue in various species (mice, pig, cat, zebrafish) and various organs (brain, lung, gut, skin, hematopoietic system; refs. 1, 2, 4–6) while maintaining antitumor efficacy equivalent to conventional radiotherapy at isodoses (1–3, 5, 7, 8). These “in vivo observations” have been termed the “FLASH effect” with postulated mechanisms of action related to redox metabolism, vascular and inflammatory responses (3). Recently, radiation oncology departments worldwide have started implementing this technology at multiple levels geared toward early phase clinical trials in humans. To this end, a feasibility study with one patient has been published (9) recently followed by a negative report (10). In the same patient with a cutaneous lymphoma, no difference in terms of side effects and tumor response when a single dose of 15 Gy when delivered at 166 Gy/s versus conventional (0.08 Gy/s) dose rate. In addition, a phase I study treating bone metastases in cancer patients with protons started in 2020 in the United States (FAST-01 trial, NCT04592887), and a phase I study on refractory metastatic melanoma (IMPULSE trial) has been started with a 9 MeV electron beam at the CHUV in 2021. Feasibility and safety in treating dog-patients with cancer with modified FLASH clinical linear accelerator (11) and proton-FLASH has also been presented (8).
Prior to the safe transfer of these findings into the clinic, certain caveats and limitations remain to be addressed. These limitations include an overreliance on mouse models, the short follow-up times reported for toxicity, use of single high doses (>10 Gy), and small volumes of irradiation (in the range of 1 cm3). Furthermore, the primary endpoint of FAST-01 clinical trial is currently focused on workflow feasibility of a palliative dose for bone metastasis. Such endpoint however is not designed to evaluate tumor cure efficacy or long-term toxicity. In the second ongoing clinical trial (IMPULSE), curative antitumor dose escalation is tested, but long-term toxicity will likely not be available, given the general palliative context of the patients. Therefore, very little is known on long-term toxicities, and our current knowledge may well underestimate possible adverse clinical outcomes known to be dependent on total dose and target volumes (12). To address the foregoing, the present study was initiated in 2018 to follow up on our previous dose escalation trial in cat patients and mini pigs (6) using our validated electron FLASH beam (eRT6, Oriatron).
This study consisted of a phase III clinical trial with cats suffering from locally advanced squamous cell carcinoma (SCC) of the nasal planum and also included a skin toxicity study on mini pigs. Three cats developed osteoradionecrosis at 9 to 15 months post-FLASH. Given this high-grade toxicity, the trial was interrupted. The pig study was designed to investigate the effects of a larger irradiation field on the skin (5–64 cm2) after FLASH exposure, and to evaluate whether a larger volume, more consistent with clinical field sizes, might modify outcomes. Field size escalation showed respectively delayed fibrotic remodeling and severe soft tissue necrosis. This study provides important data that underscores the potential limitations and caveats of FLASH-radiotherapy. Such information is a clear prerequisite for advancing safe and effective treatments to the clinic, as articulated in a recent commentary (13).
Materials and Methods
Cat patient population
For the prospective, randomized clinical phase III trial, cats with spontaneous SCCs of the nasal planum were enrolled. All cats with newly diagnosed, histologically confirmed nasal plane SCC, referred for treatment to the Division of Radiation Oncology, Vetsuisse Faculty, University of Zurich (Zurich, Switzerland) between 2019 and 2020, were eligible. Animal ethics approval statement: the study was approved by the Animal Ethics Council of the Canton of Zurich and Vaudois, Switzerland (permit numbers: ZH204/18 and VD3482). Owners' written consent was mandatory. Study termination criteria included anesthetic, animal handling complications, or side effects: VRTOG acute >grade 2 (strong) skin or mucosal toxicity (confluent moist desquamation with edema and/or ulceration, necrosis, hemorrhage or confluent fibrinous mucositis necessitating analgesia, ulceration, hemorrhage, necrosis) and VRTOG late >grade 2 skin, mucosal or bone toxicity (severe induration of skin causing physical impairment, necrosis of skin, mucosa or bone; ref. 14; Supplementary Table S1A).
Cats: treatment and follow-up
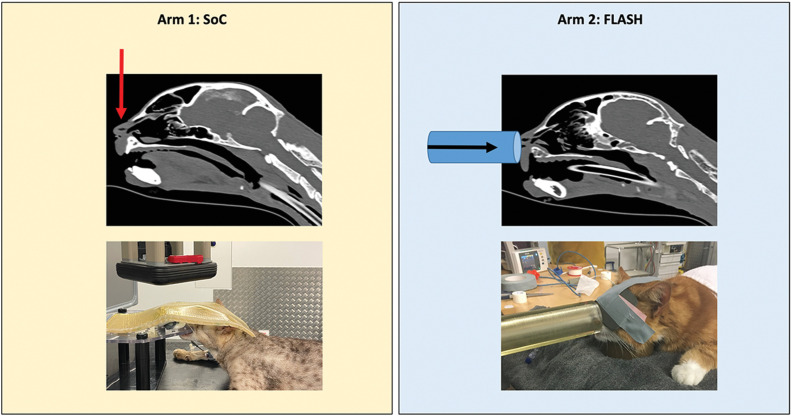
The cats were randomly assigned to one of the arms of electron radiation, with a block-randomized [equal 1:1] blinded allocation ratio. To investigate the hypothesis that a 95% tumor control rate at 1 year will be achieved with FLASH-radiotherapy, with an α-value of 0.05 and a β-value of 0.2, 29 cats needed to be included, according to power analysis (15). Arm 1 (standard-protocol group) was treated with an established accelerated protocol of 10 × 4.8 Gy (prescribed to the 90% isodose), delivered with a linear accelerator, at a dose rate of 600 MU per minute, approximately 6 Gy per minute (15, 16). We used 6, 9, or 12 MeV electrons and 4 × 4– or 6 × 6–cm field sizes, and bolus of 0.5 or 1.0 cm ensured dose build-up and homogeneity depending on the lesion treated. Arm 2 (FLASH-protocol group) was treated with 1 × 30 Gy (prescribed to the 90% isodose) FLASH-radiotherapy with eRT6/Oriatron (17) and 2.6-cm diameter tubular applicator. The 30 Gy were delivered in 20 milliseconds using three pulses, an instantaneous dose rate of 6.3 × 106 Gy/s and a mean dose rate of 1,500 Gy/s. Cats were treated under general anesthesia as previously described (15). Positioning and beam orientation are shown in Fig. 1. The single dose was chosen from a prior established maximally tolerated dose for cats (6). The patient must have been followed-up for a minimum of 3 months, preferably until death of any cause. Follow-up exams were performed under supervision of, or directly by a board-certified veterinary radiation oncologist (C. Rohrer Bley or V. Meier). The exams were performed daily in the first week (week 0), then at weeks 2, 4, 6, and 3-, 6-, 9-, 12- and 18-months postradiotherapy. Clinical visits were encouraged, but consultations via phone and photographs were an option at individual time points. The treatment response and side effects were scored along score sheets (Supplementary Table S1A and S1B). At each visit, photographs were taken and specific attention was paid to wound-healing complications, in-field fractures, vascular complications, necrosis, or second malignancies.
Figure 1.
Cat treatment set-up and positioning (arrows show beam orientation).
Dosimetry
The irradiation settings for the experiments were based on surface measurements on a 30 × 30 cm2 phantom made of solid water slabs. The phantom was placed after a polymethylmethacrylate (PMMA) tubular applicator (2.6-cm diameter) for the cat experiment. Dosimetry was performed as described in previous papers to ensure reliable and reproducible experiments (17). In addition, in vivo dosimetry was performed with a thermoluminescent dosimeter (LiF-100, Thermo Fisher Scientific) and a small Gafchromic EBT-XD film (Ashland Specialty Ingredients G.P.) placed on the skin surface of the cats in the beam to confirm the delivered dose.
Retrospective reconstruction of the dose in cats
Illustrative dose distributions in cats were calculated. Simulation of standard-of-care (SoC) treatments (arm 1) were performed using the RayStation 9A golden beam data “RSL_Clinac120” (18) with beam energies, field sizes, and boli representative of the treatment set-up. A beam model of the eRT6/Oriatron was created in the research version of the RayStation treatment planning system (RayStation 9A IONPG) using the electron Monte Carlo algorithm (ElectronMonteCarlo v3.5; ref. 18) for the FLASH arm (arm 2). That beam model was validated against EBT3 film measurements of percentage depth dose (PDD) curves and lateral profiles at multiple depths (0–30 mm) in solid water. The beam model was normalized to the measured dose per pulse at the maximum dose in water using the tubular applicator. Postradiotherapy CT scans of cats were used for arm 2 dose reconstructions because there was no CT scan available from the time of treatment. Dose distributions were simulated using a 1 × 1 × 1 mm3 scoring grid and using 10 million primary electrons per centimeter squared.
Assessment of side effects and tumor response
The (veterinary) VRTOG scoring system is not very detailed and ranges from 0 to 3, hence 3 being the most severe toxicity such as necrosis of skin or bone, or spontaneous fractures. In addition to the VRTOG toxicity criteria for cats (Supplementary Table S1A), adverse effects in both species were assessed along a detailed skin toxicity score, as prior described (6, 19). Raw data available in open repository: (https://dataverse.harvard.edu/api/access/datafile/6078697).
Tumor response was required to last for at least 21 days and noted in a modified version in adherence to RECIST; ref. 20). In case of suspected or present osteoradionecrosis a standard CT scan of the tumor patient was performed with a Brilliance CT16-slice (Philips Health Care Ltd.). Helical CT scans of the head were obtained before and 90 seconds after intravenous administration of a bolus of water-soluble iodinated contrast medium ACCUPAQUE™ 350 (GE Healthcare AG) at a dose of 700 mg/kg. The CT protocol included two reconstruction algorithms, medium frequency for soft tissue and high frequency for bone.
Statistical analysis
Data was coded in Excel (Microsoft Excel for Mac, Version 16.39) and analyzed with SPSS (IBM SPSS Statistics, Version 26, IBM Corp.). Description of quantitative data characteristics, other than time to progression (TTP) and overall survival (OS), is given by mean (±SD), unless otherwise specified. Description of qualitative characteristics is provided in absolute and relative frequencies. Differences between the two treatment arms were not calculated due to early termination of the trial. Raw data available in open repository: (https://dataverse.harvard.edu/api/access/datafile/6078697). Follow-up time was defined as the time from the first radiation treatment until death, lost to follow-up or time of data analysis. The TTP was defined as the interval between start of radiotherapy and discovery of a new or progressive lesion. Cats dying without evidence of disease progression were censored. The OS was defined as the interval between first radiotherapy until death of any cause. Cats still alive at the time of data evaluation or lost to follow-up were censored. Time to progression and OS were analyzed with Kaplan–Meier survival analysis accompanied by the log-rank or Breslow–Gehan–Wilcoxon tests. Due to the toxicity-caused early termination of the cat trial no further analyses in terms of prognostic factors could be made. Results of statistical analyses with P < 0.05 were interpreted as statistically significant.
Pigs: response of pig skin to FLASH-radiotherapy
Two female Goettingen mini pigs (43–46 kg) entered the study, and were housed at the animal research facilities of the University of Lausanne (Lausanne, Switzerland). Irradiation took place under general anesthesia. FLASH irradiation was performed using a FLASH-validated, Oriatron (eRT6, 6 MeV electrons; PMB-Alcen; ref. 21) using the parameters described in Supplementary Table S2C and dosimetry was performed as described above. Given the severe fibronecrosis produced by irradiation at conventional dose rate with the spot of 2.6 cm and reported in our previous study (6), the local ethics committee did not authorize this volume escalation study using irradiation at conventional dose rate. Therefore, FLASH irradiation only was approved and performed on the shoulder and leg of the pigs, using various applicators made of graphite or plexiglass, and with increasing size: 3.5 × 4.5 cm rectangular aperture and 8 × 8 cm square aperture in direct contact with the skin. One 8 × 8–cm area was irradiated on the PigNr 1 and two areas of each size were irradiated on the PigNr 2. The skin response to irradiation was monitored weekly through visual examination (Supplementary Table S1B), with any toxicity scored and photographed for more than 20 months’ time postirradiation. At 40 months, a skin biopsy on the large field was taken and analyzed by histology.
Dosimetry
For the pig experiments, dosimetry was performed as described above with slight modifications, the phantom was placed after a 3.5 × 4.5 cm2 graphite collimator or an 8 × 8 cm2 PMMA applicator. Dosimetry was performed as described in previous papers to ensure reliable and reproducible experiments (17). Verification was performed for the pig irradiation with Gafchromic EBT-XD film and alanine pellets (Bruker Corporation) placed on the skin surface.
Data availability
The data generated in this study are publicly available in [Harvard Dataverse] at (https://dataverse.harvard.edu/api/access/datafile/6078697).
Results
Cat-patient inclusion and treatments
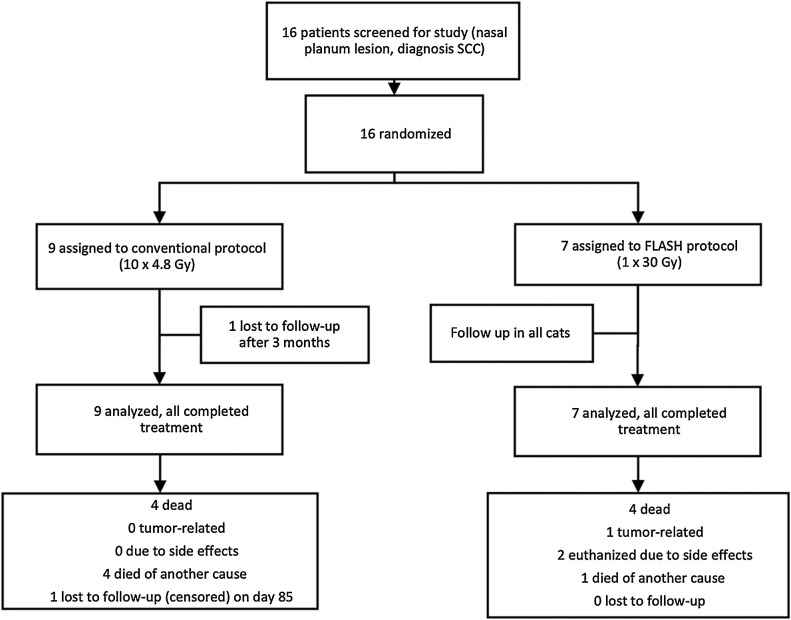
Sixteen cats were included into the prospective analysis (Fig. 2). Based on ethics approval, the occurrence of a grade 3 late toxicity was a stop-criterion for further recruitment. Thus, recruitment was stopped after 16 cats, as one of the earlier cat patients developed a grade 3 late toxicity (bone necrosis) during follow-up. Due to early termination of the trial, no further analysis was done in terms of group differences. Beam parameters are given in Supplementary Table S2A and S2B. Raw data available in open repository: (https://dataverse.harvard.edu/api/access/datafile/6078697).
Figure 2.
Trial profile.
Antitumor efficacy and OS
The mean overall follow-up time was 580 days, median 592 days [95% confidence interval (CI), 530–655]. All cats achieved complete remission (CR) and all but one cat in each group remained tumor free throughout the follow-up period. One tumor recurred in a cat from the FLASH group at 371 days postradiotherapy (validated histologically, cause of euthanasia), the other tumor recurred around 644 days postradiotherapy in a conventionally treated cat (clinical finding, no histologic exam, not cause of death). One cat's TTP observation (CatNr 1) was censored at day 401, as she received chemotherapy for an unrelated cancer. Local CR was maintained until death.
Median TTP for all cases was not reached, the mean was 890 days (95% CI, 767–1,012). Mean TTP for the conventionally treated group was 902 days (95% CI, 756–1,047) and for the FLASH-treated group 730 days (95% CI, 621–838; P = 0.51). The proportion of cats free of progression at 1 year was 100% in both groups.
At the time of analysis 8 of 16 (50%) cats were dead. One was euthanized due to local progression, 5 (31.3%) died of tumor-nonspecific causes (one of them had a clinically suspected progression, which was not cause of death), and 2 (18.8%) were euthanized due to maxillary bone necrosis. Median OS was 726 days (95% CI, 554–899). OS time did not differ: median OS for the conventionally treated group was 726 days (95% CI, 578–875), and mean OS (median not reached) for the FLASH-treated group was 656 days (95% CI, 525–793; P = 0.86).
Side effects
Acute and subacute toxicity were mild to moderate only (Table 1) and cosmetic outcome was favorable in both arms. In the conventionally treated group acute toxicity was either absent, ceased, or mild by week 6, while 1 cat treated with FLASH still had moist desquamation at week 6. Three of the 7 cats included in the FLASH arm, however, exhibited late high-grade toxicity, whereas none of the cats included in the SoC arm did. Late toxicity is listed in Table 1: a first and second cat treated with FLASH presented with mucosal breakdown in the rostral maxilla, followed by progressive bone necrosis at 12.5 (375 days, CatNr 8; Fig. 3) and 15.1 months (453 days, CatNr 6; Supplementary Fig. S1, upper panel), respectively. In both cats, bone and mucosal toxicity scored grade 3, while only grade 1 toxicity was reported at the skin level. A third cat, however, developed a severe atrophy/necrosis of the skin (lip) first, within the treated field 9.6 months (289 days, CatNr 15) after FLASH-treatment. This lesion was later followed by rostral maxillary bone necrosis at 12.9 months (387 days, CatNr 15; Supplementary Fig. S1, lower panel). While supportive treatment with nonsteroidal anti-inflammatory drugs, antibiotics as well as pentoxiphylline and vitamin E was provided, the lesions progressed. CatNr 15 had to be euthanized at the time of presentation with bone necrosis (12.9 months after FLASH therapy), CatNr 6 is still alive 319 days after the first occurrence of bone necrosis (25.7 months after FLASH therapy) with sufficient quality of life despite the bone lesion. CatNr 8 was euthanized 231 days after the first occurrence of bone necrosis (20.2 months after FLASH therapy), due to in-appetence, recurring infection, and oronasal fistula formation. In all 3 cats, the tumor on the nasal plane was in CR at the time of euthanasia.
Table 1.
Grades of acute and late side effects over time: VRTOG toxicity score (14).
| End of RT | 2 weeks post-RT | 3 months post-RT (acute) | 3 months post-RT (late) | 6 months post-RT | 9 months post-RT | 12 months post-RT | 18 months post-RT | 24 months post-RT | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | Conv (n = 9) | FLASH (n = 7) | Conv (n = 9) | FLASH (n = 7) | Conv (n = 9) | FLASH (n = 7) | Conv (n = 9) | FLASH (n = 7) | Conv (n = 8) | FLASH (n = 6) | Conv (n = 7) | FLASH (n = 7) | Conv (n = 7) | FLASH (n = 7) | Conv (n = 5) | FLASH (n = 5) | Conv (n = 4) | FLASH (n = 2) | |
| Skin | 0 | 9 | 7 | 4 | 4 | 4 | 4 | 5 | 2 | 2 | 2 | 3 | 0 | ||||||
| 1 | 3 | 4 | 5 | 3 | 5 | 3 | 3 | 3 | 5 | 4 | 4 | 2 | 5 | 1 | 2 | ||||
| 2 | 6 | 3 | 1 | 2 | 2 | 1 | 1 | ||||||||||||
| 3 | 1 | ||||||||||||||||||
| Bone | 3 | 3 | 2 | 1 | |||||||||||||||
Abbreviation: RT, radiotherapy; Conv, conventional.
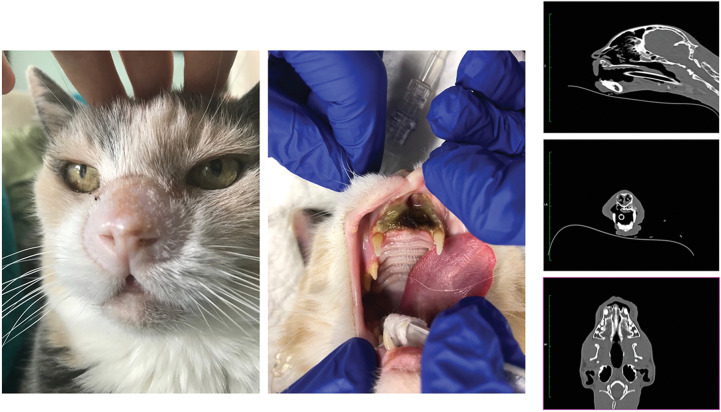
Figure 3.
Pictures show the clinical situation of the cat (CatNr 6) 14 months post-FLASH treatment. The cat presented with no external sign of complication (left) but showed maxillary and mucosal necrosis (middle). Right, Extension of the necrosis on lateral, frontal, and sagittal CT-scan sections.
Comparisons of measured and simulated PDD and lateral profiles by the eRT6 beam model are displayed in Supplementary Fig. S2. Ranges at 80% of the maximum dose and lateral full widths at 80% of the maximum dose agree within 1 mm. Supplementary Figure S3A to S3D present exemplary dose reconstructions for an arm 1 cat patient (CatNr 2), and an arm 2 cat patient (CatNr 6), which presented with bone necrosis on a CT taken 15 months postradiotherapy. This pattern is classical feature for electron beam radiotherapy in anatomical regions composed of cavities filled up with air and dense bony structure such as the nasal planum of cats. CT scans were only performed in cats presenting with osteoradionecrosis, but it would be reasonable to assume that a similar dosimetric profile would have been found in all cats, still only 3 of 7 FLASH-treated cats developed osteoradionecrosis. Despite the limitations of the linear quadratic (LQ) model applied to single dose irradiation with SoC, and the lack of empirical data sets regarding the LQ model applied to FLASH-radiotherapy, an approximation of the biological efficacy of each treatment type was evaluated using classical BED/SFED/EQD2 formula (Supplementary Table S3A–S3D). Four scenarios are reported: the first one was the SoC (10 × 4.8 Gy), the second the prescribed FLASH dose (30 Gy), the third was based upon the assumption that FLASH would provide a sparing factor of 33% (20 Gy), and the fourth based upon hotspot in the maxilla (40 Gy). Calculations showed overdosage for all the three FLASH scenarios as compared with the SoC (up to 4.5-fold for 40 Gy).
Pig study
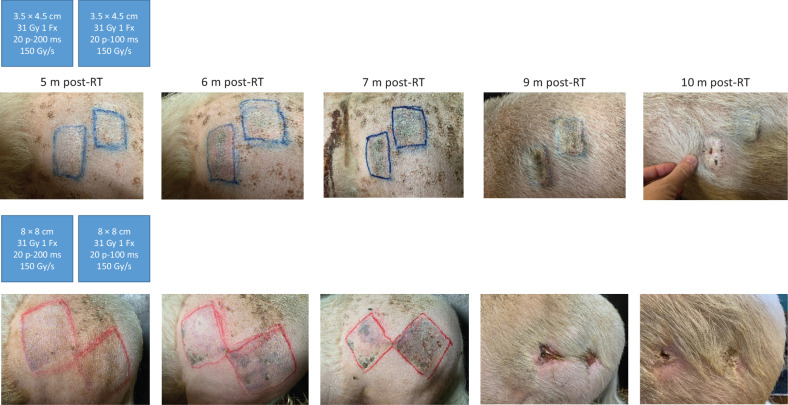
To investigate whether the beam requirements known to produce the FLASH effect in small volumes (mice) would be directly transferable to larger fields of irradiation for the same dose levels, we used a mini pig model of skin toxicity in a volume escalation study. Regardless of the volume of skin irradiated, no acute toxicity was seen by macroscopic evaluation and subacute toxicity was limited to depilation. However, late skin toxicity was found to occur in a volume-dependent manner. In our previously published experiments (6), we showed that irradiating a 2.6-cm diameter spot with a single dose of 31 Gy FLASH (10 pulses of 1.8 μs, 100 Hz, 100 milliseconds) induced minimal long-term damage, involving mainly depilation more than 12 months postirradiation and up to 5y postradiotherapy. In the same volumetric configuration, 31 Gy delivered at conventional dose rate resulted in the development of a severe fibronecrosis at 9 months postirradiation and evolving toward persistent fibro-contracture (6, 22). However, enhancing the size of the field was associated with the development of late cutaneous toxicity. With the smaller field (3.5 × 4.5 cm), late skin lesions evolved from erythema and ulceration to permanent hyperkeratosis and skin contracture at 6-, 7-, and 8 months postradiotherapy (Fig. 4). Moreover, the larger 8 × 8–cm field induced severe skin reaction with telangiectasia at 5 months postradiotherapy evolving into progressive epithelial ulceration over the entire field of irradiation at 6 months and necrotic scabs between 7 to 9 months postradiotherapy (Fig. 4). Antibiotics, pain killers, and anti-inflammatory agents were administered during the ulcerative period and ultimately the lesion healed 11 months postradiotherapy. Note that the resolution occurred by a classical wound contraction process and reepithelialization growing from the margins. In-field radiation-induced skin contracture remained stable and histopathological analysis performed 30 months postradiotherapy revealed dermal atrophy (Fig. 5).
Figure 4.
From left to right, pictures show the macroscopic evolution of skin lesions at various times post-FLASH treatment when 3.5 × 4.5–cm (shoulder) and 8 × 8–cm (leg) fields were irradiated. Blue schemes present a summary of the physical parameters used to irradiate the two adjacent areas. RT, radiotherapy; m, months.
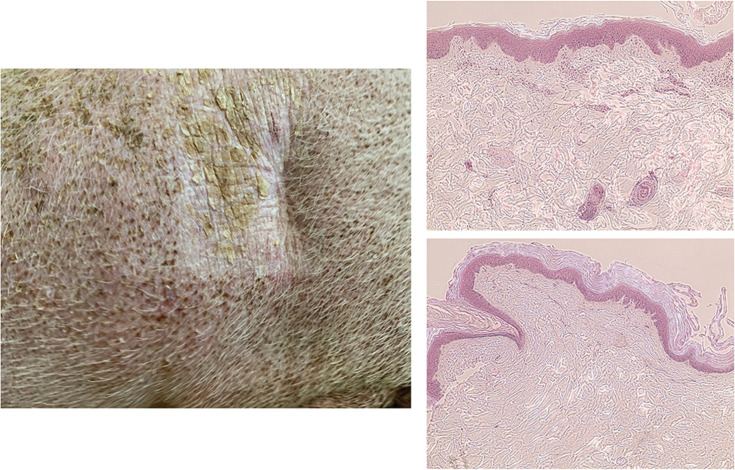
Figure 5.
Left, A picture of 8- × 8–cm field 40 months post-FLASH treatment showing persistent but fibrotic contracture and depilation. Right, Hematoxylin and eosin (H&E) histologic staining of normal skin (top, ×10) with think stratum corneum, multilayer epidermis exhibiting many papilla, and dermal appendix (vessels, muscles, hair follicles), whereas the irradiated skin (bottom, ×10) exhibits thick stratum corneum, thin epidermis lacking papilla, and dermal appendix.
Discussion
The differential sensitivity of normal tissues and tumors to FLASH-radiotherapy could provide a unique opportunity to enhance tumor control in a safe and efficacious manner. To ensure its clinical transfer, the so-called FLASH effect should be carefully investigated using appropriate models. Nowadays, many mechanistic investigations are ongoing in rodents but to better approximate clinical situations additional models are needed. In this context, a randomized phase III trial was designed to investigate the effects of FLASH single dose to a well-established SoC fractionated radiotherapy in cat patients with spontaneous tumors (15, 16). To further enhance our knowledge of more realistic clinical scenarios, we studied the normal tissue sparing capacity of FLASH-radiotherapy using larger fields of irradiation on the skin of mini pigs. Here, we selected irradiation regimes based upon of our previous experience (6) with large animals, using large single doses of irradiation (around 30 Gy) where current experiments included long-term follow-up.
The emphasis on longer-term toxicity follow-up is essential for safe clinical implementation and we have now documented the occurrence of grade 3 late bone and mucosal necrosis in some of the cats receiving single doses of 30 Gy. At conventional dose rates, such outcomes would be expected at much lower single doses, even though cats are known to not sustain a high amount of radiation damage from high-dose conventional protocols (C. Rohrer Bley's personal observation). Single fractions of up to 20 Gy are sometimes applied by veterinary radiation oncologists, but mostly in situations where only short-term outcome (palliation) is expected (C. Rohrer Bley's personal communication). However, in cats with expected long-term outcome, for example for pituitary tumors, 17 to 20 Gy were delivered in radiosurgically without obvious late toxicity (23). The herein observed osteoradionecrosis (ORN) points to realistic limitations of FLASH-radiotherapy, where a very high single dose of 30 Gy with a hotspot at 42 Gy exceeded the tolerance of the bone maxilla and the oral mucosa in cats, despite only grade 1 toxicity reported for skin. This differential tissue tolerance suggests that organ-specific dose-response studies are needed to identify the MTD for a given tumor bed through controlled dose escalation and when necessary (i.e., bone and mucosa) dose deescalation studies. In any case, current findings highlight the need for caution when implementing FLASH at higher doses/fraction in the clinic. In addition, and despite the aggressive radiotherapy protocols used, a new tumor occurrence or a relapse were observed over 1 year posttreatment in 1 cat from each arm. As previously reported by us in human acute lymphoblastic leukemia (4), interindividual variations in radioresponse are likely to extend over vastly different dose rate paradigms and remain to be investigated. However, these data in cat patients provide the first long-term tumor remission results ever published and support no tumor protection induced by FLASH-radiotherapy as shown in tumor growth delay experiments in mice. In addition to the study in cat patients, the volume-dependent late skin toxicity findings in mini pigs at an isodose of 31 Gy in FLASH mode point to the need for studies focused on evaluating the impact of larger field sizes.
For decades, dose fractionation protocols have been optimized to protect normal tissues. In this study by design, we incorporated several high-risk factors known to precipitate normal tissue complications including single, very high dose (>30 Gy) irradiation and larger field sizes of surface exposure. Despite the use of FLASH-radiotherapy at 150 Gy/s, severe necrosis was induced in pig skin. Unfortunately, we were not allowed to compare this outcome with a similar isodose delivered at conventional dose rates, based on prior data documenting severe ulcerations obtained with a spot size of 2.6 cm, precluding ethical approval of large field treatments at conventional dose rates. Therefore, we can only speculate that if 31 Gy was delivered using these larger fields at conventional dose rate, fibronecrosis would have occurred much earlier, been more severe, and/or would not have resolved over time or even have extended outside target volume. The foregoing is however, supported by previous studies performed in pig skin irradiated with conventional dose rates (24, 25). In those studies, field size was shown to influence the dose required to induce acute skin reactions. For late skin reactions however, it was true only for smaller fields (<10 mm), whereas with larger field sizes the ED50% was independent of the area exposed. The authors concluded that for anatomical/structural radiation damage, field size is an absolute concept, and that no scaling factor can be related to animal size (24).
In cats where ORN was induced in 3 of 7, the target volume was small (2.6 cm), the dose rate was ultra-high (1,500 Gy/s), and was delivered in a single fraction (30 Gy with hot-spots up to 42 Gy). As compared with the absence of late effects in the SoC arm (10 × 4.8 Gy), the adverse outcomes in the FLASH arm can, at first approximation, be attributed to the significant imbalance between the biologically effective dose of the two arms of the study, as shown Supplementary Table S3A and S3B. In human patients treated for carcinoma of the tonsil, field size is not relevant for mucosal breakdown, but is a significant factor for mandibular (bone) complications. The estimated α/β ratio for bone is 0.85 Gy (−0.48, 2.4 Gy) summarized in ref. 26, rendering dose per fraction a most important factor, as this value falls at the lower end of estimated α/β ratios when compared with other late responding normal tissues. While bone possesses the radiobiologic characteristics of normal tissue that exhibit heightened sensitivity to increased dose per fraction (26), other factors such as oral hygiene, dental status or postradiation trauma such as tooth extraction could further exacerbate normal tissue sequelae. Our study design was based upon our previous data from cats, indicating that a single dose between 28 to 34 Gy was sufficient to safely eradicate tumors. Although this initial study was performed with only 1 cat at each dose level, complications were not encountered during the 1 year follow-up, but longer-term follow-up was missing (6). Unfortunately, neither preclinical data obtained in mice nor the phase I trial performed on a small group of cats was sufficiently predictive of this toxicity, for reasons that might include the issue of a too short follow-up period in the former trial.
Notwithstanding the continued promise of FLASH-radiotherapy, optimization of beam, and other treatment parameters remains a priority. In cats, the SoC-protocol was administered using a vertical beam and a bolus, whereas the administration of the 30 Gy FLASH-protocol was frontal (Fig. 1) and performed without any bolus. Given the DDP of the electron beam provided by the eRT6/Oriatron (17), hotspots (125% of the dose) occurred at the maxilla level as shown by our retrospective dosimetric reconstruction (Supplementary Fig. S3). However, while this electron pattern of dose deposition occurred in all 7 FLASH-treated cats, only 3 of them developed severe complications, which suggest a sparing action of FLASH-radiotherapy despite the high single-dose protocol used. A lateral angle (cat position in lateral decubitus) could have been implemented to optimize the conformality of our FLASH-treatment given the fixed horizontal beam geometry. In this light, technological development remains a critical factor that needs to be properly implemented to realize the latest developments of radiotherapy in terms of ballistics, imaging, and treatment planning systems with FLASH capabilities.
While the precise FLASH beam requirements to produce the FLASH effect are still under investigation, we used a well-characterized FLASH-validated beam to perform the current investigations (eRT6/Oriatron). The role of the temporal beam structure is critical, and studies in mice using whole-brain and whole-abdomen irradiation protocols and in zebrafish embryos using total embryo irradiation have found that the optimal normal tissue sparing effect was obtained using a single electron pulse (over microsecond timescales; refs. 3, 27–30). These data suggest that shortening the time of irradiation as much as possible would provide the most likely scenario to produce the FLASH effect. Therefore, our cat treatment protocol was performed with a dose rate of 1,500 Gy/s to decrease the overall time of irradiation as much as possible. This dose rate was 5-fold higher than in our previous phase I clinical trial (300 Gy/s) and decreased the time of irradiation by 10-fold. Ideally, a one-pulse delivery of 30 Gy would have been selected but was technically not feasible. Consequently, the total dose was delivered in three pulses over 20 milliseconds. As a result, we cannot formally exclude this slight increase in overall treatment time to be responsible in part for the observed bone toxicity. Whether the average dose rate was too low as compared with one pulse or too high as compared with 300 Gy/s remains uncertain. An individual and specific radio-sensitivity of the three cats with complications cannot be excluded either. Interestingly, a single 30-Gy FLASH dose seems to be tolerated by both cats and pigs at the skin surface with a 2.6-cm diameter field. In pigs, the tolerance of the skin was volume-dependent, but the parametric limitations imposed by the irradiator itself necessitated an increase in overall treatment time. Hence, to irradiate large fields with a single high dose of 31 Gy and maintain flatness, beam parameters had to be adjusted. The 31-Gy FLASH dose was delivered in 20 pulses of 1.8 μs at 100 Hz, that doubled the time of irradiation and may have impacted skin necrosis. These results suggest that direct transposition of preclinical parameters is not straightforward, as the magnitude of the FLASH sparing effect could be reduced when larger irradiation fields are used and needs to be carefully investigated. Alternatively, larger irradiation fields are likely to impose more demanding requirements on the FLASH beam itself, which would require further refinement to optimize normal tissue protection. Despite of our small animal number and the possible inherent susceptibility of individual animals to radiation damage, differences in volume, species, and organ responses should not be overlooked, and must be evaluated in tandem with more accurate and systematic definitions of the time signature of beams and accompanying technology. However, we acknowledge further limitations to this study:
i. Patient number is small, and the randomized controlled trial had to be preliminary terminated due to inacceptable side effects. Yet, we deem our findings of relevance as the long follow-up period managed to show a strong difference in late toxicity between the protocols, after comparably mild acute side effects.
ii. Radiation regimen (fractionation) was different in the FLASH-radiotherapy and SoC arms as the FLASH-protocol was based on our previous dose escalation study and not on biological effectiveness calculation. Nevertheless, the 30-Gy FLASH resulted in very high local control, indicating that FLASH does not result in a protective effect on cancer cells, a theoretical concern voiced before. So far conventional fractionation experiments at ultra-high dose rate have never been performed and are today a priority for the clinical translation of FLASH-radiotherapy. If conventional fractionation is FLASH-compatible, step-by-step fraction escalation starting from the SoC would be the safest way to validate FLASH benefit in human patients. If conventional fractionation is not possible, other protocols should be investigated with hypofractioned regimen or hybrid protocols (delivering FLASH-radiotherapy as a boost).
iii. Pig skin experiments were also small, but they show a clear dependence of late toxicity on the volume irradiated. Note that the resolution occurred by a classical wound contraction process and reepithelialization growing from the margins. The magnitude of the FLASH sparing effect could therefore be less when larger irradiation fields are used.
In conclusion, our study is the first to shed light on certain caveats in the path toward clinical translation of FLASH-radiotherapy and shows that implementation of single-high-dose and large field irradiations will present challenges for minimizing long-term toxicities even with FLASH dose rates. We believe that clinical trials with domestic animal patients (cats and dogs) are safe and quick way to investigate FLASH-radiotherapy benefit and avoid possible failure in human clinical trial. At the technological level, implementation of state-of-the art ballistics, imaging and treatment plan should be coupled with FLASH capabilities and systematic characterization of the beam parameters will be required to unravel the full potential of FLASH-radiotherapy, which remains a significant hurdle with existing technology.
Supplementary Material
Acknowledgments
The authors thank Prof. S. Delanian for helpful discussion in the management of ORN; Dr. Erik Traneus from RaySearch Labs for the creation of the eRT6 beam model in RayStation; and Profs. M. Ozsahin and F. Bochud and Drs. J.F. Germond and C. Bailat for input regarding dosimetric reconstructions and fruitful scientific discussions. We also thank G. Pavillard for pig caretaking and M. Santos, S. Zufferey Pidoux, and C. Moratal from Institute of Radiation Physics (IRA; University Hospital and University of Lausanne, Lausanne, Switzerland). The cat study was supported by the Krebsliga KFS-4438-02-2018. P. Gonçalves Jorge was partly supported by NIH grant P01CA244091-01 and I. Petridis by MAGIC- FNS CRS II5_186369; both were partly supported by ISREC Foundation thanks to a donation from the Biltema Foundation. The pig study was supported by ISREC Foundation thanks to a donation from the Biltema Foundation.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors’ Disclosures
C. Rohrer Bley reports grants from Krebsliga during the conduct of the study. V. Meier reports grants from Krebsliga during the conduct of the study. M.-C. Vozenin reports grants from Krebsliga, Swiss National Science Foundation, ISREC Foundation, and NIH during the conduct of the study. No disclosures were reported by the other authors.
Authors’ Contributions
C. Rohrer Bley: Conceptualization, resources, data curation, formal analysis, supervision, funding acquisition, investigation, methodology, writing–original draft, project administration, writing–review and editing. F. Wolf: Data curation, investigation, writing–original draft, writing–review and editing. P. Gonçalves Jorge: Conceptualization, data curation, formal analysis, investigation, writing–original draft, writing–review and editing. V. Grilj: Data curation, formal analysis, investigation, writing–original draft, writing–review and editing. I. Petridis: Investigation, writing–original draft, writing–review and editing. B. Petit: Investigation, writing–original draft, writing–review and editing. T.T. Böhlen: Resources, data curation, formal analysis, investigation, visualization, writing–original draft, writing–review and editing. R. Moeckli: Resources, formal analysis, investigation, visualization, writing–original draft, writing–review and editing. C. Limoli: Conceptualization, resources, supervision, funding acquisition, writing–original draft, writing–review and editing. J. Bourhis: Conceptualization, supervision, writing–original draft, writing–review and editing. V. Meier: Investigation, writing–original draft, writing–review and editing. M.-C. Vozenin: Conceptualization, resources, data curation, formal analysis, supervision, funding acquisition, validation, investigation, visualization, methodology, writing–original draft, project administration, writing–review and editing.
References
- 1. Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, Fouillade C, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med 2014;6:245ra93. [DOI] [PubMed] [Google Scholar]
- 2. Montay-Gruel P, Acharya MM, Goncalves Jorge P, Petit B, Petridis IG, Fuchs P, et al. Hypofractionated FLASH-RT as an effective treatment against glioblastoma that reduces neurocognitive side effects in mice. Clin Cancer Res 2021;27:775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kacem H, Almeida A, Cherbuin N, Vozenin MC. Understanding the FLASH effect to unravel the potential of ultra-high dose rate irradiation. Int J Radiat Biol 2022;98:506–16. [DOI] [PubMed] [Google Scholar]
- 4. Chabi S, To THV, Leavitt R, Poglio S, Jorge PG, Jaccard M, et al. Ultra-high-dose-rate FLASH and conventional-dose-rate irradiation differentially affect human acute lymphoblastic leukemia and normal hematopoiesis. Int J Radiat Oncol Biol Phys 2021;109:819–29. [DOI] [PubMed] [Google Scholar]
- 5. Levy K, Natarajan S, Wang J, Chow S, Eggold JT, Loo PE, et al. Abdominal FLASH irradiation reduces radiation-induced gastrointestinal toxicity for the treatment of ovarian cancer in mice. Sci Rep 2020;10:21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vozenin MC, De Fornel P, Petersson K, Favaudon V, Jaccard M, Germond JF, et al. The advantage of FLASH radiotherapy confirmed in mini-pig and cat-cancer patients. Clin Cancer Res 2019;25:35–42. [DOI] [PubMed] [Google Scholar]
- 7. Cunningham S, McCauley S, Vairamani K, Speth J, Girdhani S, Abel E, et al. FLASH proton pencil beam scanning irradiation minimizes radiation-induced leg contracture and skin toxicity in mice. Cancers 2021;13:1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Velalopoulou A, Karagounis IV, Cramer GM, Kim MM, Skoufos G, Goia D, et al. FLASH proton radiotherapy spares normal epithelial and mesenchymal tissues while preserving sarcoma response. Cancer Res 2021;81:4808–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bourhis J, Sozzi WJ, Jorge PG, Gaide O, Bailat C, Duclos F, et al. Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol 2019;139:18–22. [DOI] [PubMed] [Google Scholar]
- 10. Gaide O, Herrera F, Sozzi WJ, Goncalves Jorge P, Kinj R, Bailat C, et al. Comparison of ultra-high versus conventional dose rate radiotherapy in a patient with cutaneous lymphoma. Radiother Oncol 2022. [DOI] [PubMed] [Google Scholar]
- 11. Konradsson E, Arendt ML, Bastholm Jensen K, Borresen B, Hansen AE, Back S, et al. Establishment and initial experience of clinical FLASH radiotherapy in canine cancer patients. Front Oncol 2021;11:658004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vozenin MC, Montay-Gruel P, Limoli C, Germond JF. All irradiations that are ultra-high dose rate may not be FLASH: the critical importance of beam parameter characterization and in vivo validation of the FLASH effect. Radiat Res 2020;194:571–2. [DOI] [PubMed] [Google Scholar]
- 13. Buchsbaum JC, Coleman CN, Espey MG, Prasanna PGS, Capala J, Ahmed MM, et al. FLASH radiation therapy: new technology plus biology required. Int J Radiat Oncol Biol Phys 2021;110:1248–9. [DOI] [PubMed] [Google Scholar]
- 14. Ladue T, Klein MK, Veterinary Radiation Therapy Oncology Group. Toxicity criteria of the veterinary radiation therapy oncology group. Vet Radiol Ultrasound 2001;42:475–6. [DOI] [PubMed] [Google Scholar]
- 15. Gasymova E, Meier V, Guscetti F, Cancedda S, Roos M, Rohrer Bley C. Retrospective clinical study on outcome in cats with nasal planum squamous cell carcinoma treated with an accelerated radiation protocol. BMC Vet Res 2017;13:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Melzer K, Guscetti F, Rohrer Bley C, Sumova A, Roos M, Kaser-Hotz B. Ki67 reactivity in nasal and periocular squamous cell carcinomas in cats treated with electron beam radiation therapy. J Vet Intern Med 2006;20:676–81. [DOI] [PubMed] [Google Scholar]
- 17. Jorge PG, Jaccard M, Petersson K, Gondre M, Duran MT, Desorgher L, et al. Dosimetric and preparation procedures for irradiating biological models with pulsed electron beam at ultra-high dose-rate. Radiother Oncol 2019;139:34–9. [DOI] [PubMed] [Google Scholar]
- 18. RaySearch_Laboratories. RayStation 9A Reference Manual. 2019.
- 19. Daburon F. Irradiations aigues localisées: Bases radiobiologiques du diagnostic et du traitement. Paris, France: EDP Sciences; 1997. [Google Scholar]
- 20. Nguyen SM, Thamm DH, Vail DM, London CA. Response evaluation criteria for solid tumours in dogs (v1.0): a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol 2015;13:176–83. [DOI] [PubMed] [Google Scholar]
- 21. Jaccard M, Duran MT, Petersson K, Germond JF, Liger P, Vozenin MC, et al. High dose-per-pulse electron beam dosimetry: commissioning of the Oriatron eRT6 prototype linear accelerator for preclinical use. Med Phys 2018;45:863–74. [DOI] [PubMed] [Google Scholar]
- 22. Schuller A, Heinrich S, Fouillade C, Subiel A, De Marzi L, Romano F, et al. The European Joint Research Project UHDpulse - Metrology for advanced radiotherapy using particle beams with ultra-high pulse dose rates. Phys Med 2020;80:134–50. [DOI] [PubMed] [Google Scholar]
- 23. Watson-Skaggs ML, Gieger TL, Yoshikawa H, Nolan MW. Endocrine response and outcome in 14 cats with insulin resistance and acromegaly treated with stereotactic radiosurgery (17 Gy). Am J Vet Res 2021;83:64–71. [DOI] [PubMed] [Google Scholar]
- 24. Hopewell JW, Trott KR. Volume effects in radiobiology as applied to radiotherapy. Radiother Oncol 2000;56:283–8. [DOI] [PubMed] [Google Scholar]
- 25. Lefaix JL, Daburon F. Diagnosis of acute localized irradiation lesions: review of the French experimental experience. Health Phys 1998;75:375–84. [DOI] [PubMed] [Google Scholar]
- 26. Withers HR, Peters LJ, Taylor JM, Owen JB, Morrison WH, Schultheiss TE, et al. Late normal tissue sequelae from radiation therapy for carcinoma of the tonsil: patterns of fractionation study of radiobiology. Int J Radiat Oncol Biol Phys 1995;33:563–8. [DOI] [PubMed] [Google Scholar]
- 27. Montay-Gruel P, Bouchet A, Jaccard M, Patin D, Serduc R, Aim W, et al. X-rays can trigger the FLASH effect: Ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother Oncol 2018;129:582–8. [DOI] [PubMed] [Google Scholar]
- 28. Montay-Gruel P, Petersson K, Jaccard M, Boivin G, Germond JF, Petit B, et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiother Oncol 2017;124:365–9. [DOI] [PubMed] [Google Scholar]
- 29. Ruan JL, Lee C, Wouters S, Tullis IDC, Verslegers M, Mysara M, et al. Irradiation at ultra-high (FLASH) dose rates reduces acute normal tissue toxicity in the mouse gastrointestinal system. Int J Radiat Oncol Biol Phys 2021;111:1250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vozenin MC, Hendry JH, Limoli CL. Biological benefits of ultra-high dose rate FLASH radiotherapy: sleeping beauty awoken. Clin Oncol (R Coll Radiol) 2019;31:407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are publicly available in [Harvard Dataverse] at (https://dataverse.harvard.edu/api/access/datafile/6078697).