Abstract
Background
Vaccination has been promoted to control viral transmission in response to the coronavirus disease 2019 (COVID-19) pandemic. Cases of new-onset or exacerbation of psoriasis, an immune-mediated inflammatory disease, were reported following COVID-19 vaccination. However, a comprehensive review examining the association between COVID-19 vaccination and the occurrence or exacerbation of psoriasis has yet to be performed.
Objective
The aim of this systematic review is to investigate the demographics, clinical variables, and outcomes associated with psoriasis following COVID-19 vaccination.
Methods
A systematic literature search was conducted using the PubMed, Embase, Web of Science, and Cochrane databases from database inception to April 25, 2022. The review included studies with relevant terms, including ‘psoriasis,’ ‘psoriasis vulgaris,’ ‘guttate psoriasis,’ ‘pustular psoriasis,’ ‘palmoplantar pustulosis,’ ‘psoriatic erythroderma,’ ‘psoriatic arthritis,’ ‘COVID-19,’ and ‘vaccine.’ We included all studies reporting at least one patient who developed new-onset psoriasis or experienced a psoriasis flare following at least one dose of any COVID-19 vaccine. A flare was defined as the worsening of disease conditions after vaccination according to the study by Gregoire et al. The appraisal tool described by Murad et al. was used to assess the quality of case reports and series, whereas the National Institute of Health quality assessment tool was used to assess observational studies.
Results
The initial search yielded 367 results, including 7 studies reporting new-onset psoriasis, 32 studies reporting psoriasis flares, and 4 studies reporting both. The most commonly observed psoriasis subtype was plaque-type psoriasis. mRNA vaccines, including those produced by Moderna and BioNTech/Pfizer, were frequently associated with subsequent psoriasis episodes. First, second, and third vaccine doses were associated with psoriasis incidents, with the second dose most frequently associated with psoriasis flares. Delayed onset was observed, ranging from 2 to 21 days in the new-onset group and from 1 to 90 days in the flare group. Most patients experienced favorable outcomes, with improvement or resolution occurring within 3 days to 4 months.
Conclusions
Both new-onset psoriasis and psoriasis flares were reported as cutaneous adverse events following COVID-19 vaccination. Psoriatic patients may require regular follow-up before and after COVID-19 vaccination.
Trial Registration
Review registration number PROSPERO database: CRD42022304157.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40257-022-00721-z.
Key Points
| This systematic review identified all COVID-19 vaccines associated with psoriasis onset, with mRNA vaccines, including those produced by Moderna and BioNTech/Pfizer, frequently associated with subsequent psoriasis episodes. |
| First, second, and third vaccine doses were reported to induce psoriasis, with the second dose most commonly associated with psoriasis flares. |
| Delayed onset was observed, ranging from 2 to 21 days in the new-onset group and from 1 to 90 days in the flare group. |
| Both new-onset psoriasis and psoriasis flares are possible cutaneous adverse events following COVID-19 vaccination. |
Introduction
Globally, the novel coronavirus disease 2019 (COVID-19) pandemic has drastically impacted general health and the economy. Vaccines have been developed to protect against infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), representing one of the most reliable methods for controlling viral transmission [1]. Commonly used vaccines developed for COVID-19 include mRNA vaccines, such as Comirnaty® (BioNTech/Pfizer; BNT162b2) and Spikevax® (Moderna; mRNA-1273), which encode the full-length SARS-CoV-2 spike protein; chimpanzee adenovirus vector vaccine, such as Covishield® (AstraZeneca; AZD1222/ChAdOx1); and inactivated viral protein vaccines, such as CoronaVac (Sinovac). COVID-19 vaccines have achieved wide coverage, with 60.8% of the global population having received at least one vaccine dose as of January 2022 [1, 2]. Increasing reports have described cutaneous adverse events (CAEs) related to COVID-19 vaccination, which have been considered to be vaccine-related new-onset cases or flares of pre-existing dermatoses [1, 3–5]. Various CAEs have been documented following vaccination, including injection-site reactions, morbilliform rash, urticaria, pityriasis rosea, psoriasis, and bullous pemphigoid [6–14].
Psoriasis is a commonly occurring inflammatory disease that affects 0.5−11.4% of the adult population worldwide and involves a complex pathogenic mechanism linked to several immune cells and cytokines, including tumor necrosis factors, interleukin (IL)-17, IL-22, and IL-23 [15–18]. Various psoriasis subtypes have been defined, including plaque, guttate, pustular, and nail psoriasis and psoriatic arthritis (PsA) [19]. The safety and efficacy of COVID-19 vaccines have been demonstrated among psoriasis patients treated with systemic therapies [20–23], and consensus guidelines have been established regarding the vaccination timing of individuals treated with systemic immunomodulatory medications to optimize the vaccine response [24, 25]. However, increasing reports describe the development of new-onset psoriasis or psoriasis exacerbations with an increase in the total population of vaccinated individuals [26]. Patients who experience psoriatic events following vaccination, regardless of any prior history of diagnosis or treatment, can present with diverse clinical manifestations [27–29]. To our knowledge, no comprehensive review has examined the issues surrounding COVID-19 vaccination-related psoriasis. We conducted this systematic review to provide an overview of the demographic and clinical factors and outcomes associated with psoriasis episodes following COVID-19 vaccination.
Materials and Methods
This meta-analysis was registered in PROSPERO (CRD42022304157) and was performed in accordance with the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [30]. The PubMed, Embase, Cochrane Library, and Web of Science databases were searched for relevant studies from their respective inception to April 25, 2022. Relevant terms, including ‘psoriasis,’ ‘psoriasis vulgaris,’ ‘guttate psoriasis,’ ‘pustular psoriasis,’ ‘palmoplantar pustulosis,’ ‘psoriatic erythroderma,’ ‘psoriatic arthritis,’ ‘COVID-19,’ and ‘vaccine’ were used as free text, medical subject headings (MeSH in PubMed and Emtree in Embase), and abbreviations in the literature search. Keywords were combined using appropriate Boolean operators, and a primary search strategy was developed without limitations regarding language or published data (Table S1, see electronic supplementary material [ESM]). In addition, the reference lists of all articles identified during the database search were examined to identify other potentially relevant articles.
We included studies reporting at least one patient who developed new-onset psoriasis or experienced a psoriasis flare following at least one dose of any COVID-19 vaccine. We defined a flare after vaccination as the presence of one or more of the following according to the study by Gregoire et al. [31]: documented increases of body surface area involvement, higher psoriasis area and severity index score (PASI), presence of pustular or erythrodermic skin, patient subjective report of worsening, physical examination results describing worsening psoriasis compared with a previous examination, and clinician assessment or plan with mention of flare, rebound, or worsening psoriasis. Review articles, conference abstracts, and studies conducted using in vitro or animal models were excluded. Two experienced reviewers (Wu and Huang) independently performed the literature search, data extraction, and quality assessments. Any discrepancies between the two reviewers were resolved by a third reviewer (Chen). The appraisal tool described by Murad et al. was used to assess the quality of case reports and series, whereas the National Institute of Health quality assessment tool was used to evaluate observational studies (Table S2, see ESM) [32].
The following data were extracted independently by two reviewers (Wu and Huang) from the included studies: author, year of publication, country, patients’ demographic information (age, sex, medical history), COVID-19 vaccination information (vaccine type, dose), onset time, whether cases were new onsets or flares, psoriasis subtype, other possible triggers, skin manifestations, laboratory study, treatment (prior treatment and treatment after flares), outcomes, and reactions to further COVID-19 vaccination. We further classified patient groups according to the occurrence of new psoriasis onset or psoriasis flares and categorized all patients according to the psoriasis subtype.
Results
Literature Search
As shown in Fig. 1, 367 studies were identified after searching four major databases and performing a manual search of the reference lists of identified studies. We excluded 172 studies as duplicates, and 93 studies were excluded for being unrelated to the study question after assessing the title or abstract. The full texts of the remaining 102 studies were reviewed, and 43 studies were identified as meeting the inclusion criteria for qualitative synthesis. A total of 7 studies reporting new-onset psoriasis, 32 studies reporting psoriasis flares, and 4 studies reporting both new-onset psoriasis and psoriasis flares were included in this study (Tables 1, 2).
Fig. 1.
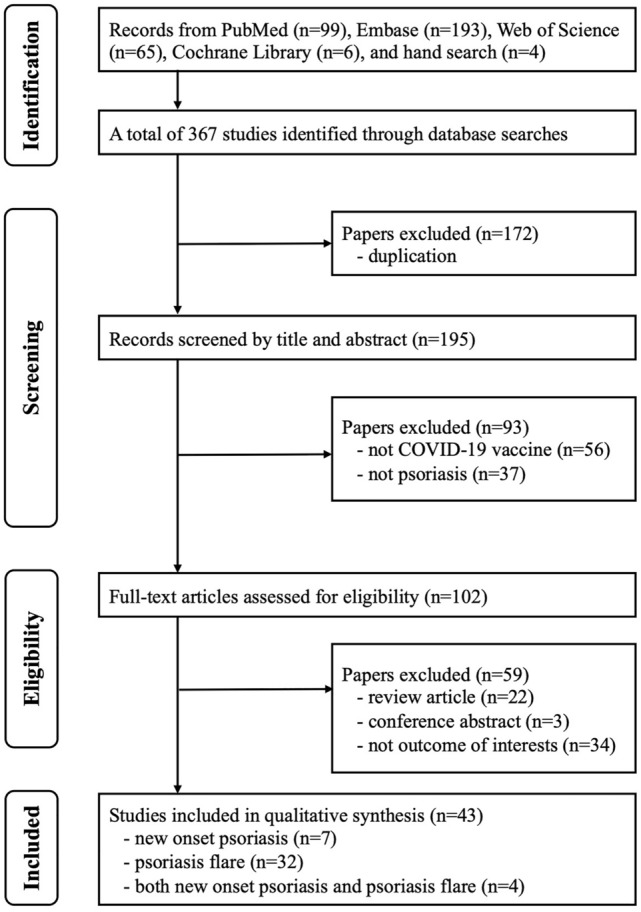
PRISMA flowchart of the selection of studies
Table 1.
Characteristics of the included studies reporting new onset psoriasis
| Study, Country | Age, sex | History | Vaccine (dose) | Onset | Other triggers | Skin manifestations | Laboratory study | Treatment | Outcome (time) | Further vaccine |
|---|---|---|---|---|---|---|---|---|---|---|
| Plaque | ||||||||||
| McMahon et al. 2022 [14] USA | 67 NR | NR | Moderna, NR | NR | NR | Well demarcated erythematous papules and plaques with overlying silvery scale over trunk, extremities, head, neck and face | NR | NR | NR | NR |
| Wei et al. 2022 [9] USA | 16 pt | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Guttate | ||||||||||
| Lehmann et al. 2021 [29], Switzerland | 79 F | T2DM and HTN | Pfizer (1st) | 10 d | No prior or family history of psoriasis or any other putative triggers (new intake of med, underlying infections) | Numerous, disseminated, erythematous papules and partly scaly plaques mainly on the extensor surface of her arms, thighs, back and scalp at first dose. A flare-up particularly on her arms and legs at second dose | NR | TCS/TCAL, UVB PT | Improved (NR) | Flare after the 2nd dose of Pfizer |
| Magro et al. 2021 [34] USA | 58 M | NR | Pfizer (2nd) | 14 d | NR | Red spots all over the body | NR | NR | Resolved (NR) | None |
| Pesqué et al. 2022 [28] Spain | 72 M | IgG-k MM treated with AHPCT | Moderna (2nd) | 6 d | No new drugs had been commenced and the patient denied any other possible triggers | Multiple erythematous, non-confluent papules, 3–15 mm in diameter with silvery-white desquamation involving trunk and extremities | Normal BR, elevated CRP, throat culture (-), normal titers of ASLO, HIV (-), T. pallidum (-) | TCS/TCAL | Resolved (NR) | None |
| Song et al. 2022 [33] Korea | 23 F | None | Pfizer (1st) | 2 d | No personal or family history of psoriasis. No med history | Drop-like scaly erythematous papules and plaques affecting the trunk, the extremities, and the buttock | Normal BR, CRP, ESR, and ASLO titer | TCS/TCAL | Improved (2 wk) | Flare after the 2nd dose |
| Wei et al. 2022 [9] USA | 6 pt | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| GPP | ||||||||||
| Elamin et al. 2022 [36] UK | 66 F | HTN and depression | AZ (1st) | 21 d | No prior dermatological issues and no family history of psoriasis or other skin diseases. No new med had been commenced in the weeks preceding the rash and no recent illnesses | Extensive erythematous pustular rash to the trunk and proximal aspect of the limbs, with no mucosal membrane or palmoplantar involvement | Normal BR | OAC 20 mg once daily, TCS | Resolved (NR) | No flare after the 2nd dose |
| Romagnuolo et al. 2022 [35] Italy | 64 F | Well-controlled SLE | Pfizer (1st) | Days | NR | Multiple erythemato-violaceous, annular-polycyclic, slightly infiltrated and scaling lesions distributed on her trunk, neck and limbs. Pustules were visible in some lesions | NR | MTX 15 mg QW | Improvement (1 mo) | Flare after the 2nd dose |
| Nail psoriasis | ||||||||||
| Ricardo and Lipner 2021 [27] USA | 76 F | OA affecting both hands and asthma | Pfizer (2nd) | 7 d | History of asymptomatic SARS-CoV-2 infection 6mo prior to vaccination confirmed by positive COVID-19 PCR and Ab test results. She denied previous manicuring, trauma, or med that caused onycholysis | Severe distal onycholysis, subungual hyperkeratosis, nail pitting, oil drops, splinter hemorrhages, and proximal nailfold erythema affecting multiple fingernails. Degenerative changes of the small joints of both hands (NAPSI 18) | NR | TCS | Improved (2m) | None |
| Not specified | ||||||||||
| Nagrani et al. 2021 [5] India | 65 F | None | AZ (2nd) | 10 d | No previous history of psoriasis | Scaly erythematous papules and plaques over trunk and extremities (BSA 30%) | NR | APR (10 mg on day 1, 30 mg BID by day 7), OAH, emollients | Improved (7 d) | None |
| Català et al. 2022 [37] Spain | 3 pt | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Wei et al. 2022 [9] USA | 89 M | NR | Moderna (2nd) | 24 d | No previous COVID-19 infection | Over scalp, torso, arms, legs (BSA 60%) | NR | IXE, OAC 25 mg | Resolved (NR) | None |
Ab antibody, AHPCT autologous hematopoietic progenitor cell transplantation, APR apremilast, ASLO antistreptolysin O, AZ AstraZeneca, BID twice daily, BR blood routine, BSA body surface area, COVID-19 coronavirus disease, CRP C-reactive protein, d day, ESR erythrocyte sedimentation rate, GPP generalized pustular psoriasis, HIV human immunodeficiency virus, HTN hypertension, IXE ixekizumab, med medication, MM multiple myeloma, mo month, MTX methotrexate, NAPSI Nail Psoriasis Severity Index, NR not reported, OA osteoarthritis, OAC oral acitretin, OAH oral antihistamine, OCS oral corticosteroids, PASI Psoriasis Area and Severity Index, PCR polymerase chain reaction, pt patient(s), PT phototherapy, QD once daily, QW once weekly, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, SLE systemic lupus erythematosus, T2DM type 2 diabetes mellitus, TCAL topical calcipotriol, TCS topical corticosteroids, UVB ultraviolet B, wk week
Table 2.
Characteristics of the included studies reporting psoriasis flare
| Study, country | Age, sex | History | Vaccine (dose) | Onset | Other triggers | Skin manifestations | Laboratory study | Prior tx | Tx after flare | Outcome (time) | Further vaccine |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Plaque | |||||||||||
| Bostan et al. 2021 [41] Turkey | 51 M | T2DM, psoriasis for 1 y | Pfizer (both) | 14 d | No recent history of infection, med, or stress | Confluent, erythematous, scaly, thick plaques covering the entire knees, upper extremities, buttocks, and extending to the trunk, both thighs and legs (PASI 27.1) | Normal BR and biochemistry. Slightly elevated CRP level (0.99 mg/dL) | TCS | NR | NR | Flare after both doses of Pfizer |
| Turkey | 52 M | Occasional scattered rash with a remitting-relapsing course over the past 5 y | Sinovac (2nd) | 30 d | No recent history of infection, med, or stress | Erythematous, scaly plaques, some of which had a targetoid appearance, located on the upper and lower extremities, nape of the neck and trunk (PASI 20.3) | Normal BR | NR | NR | NR | None |
| Chao and Tsai 2021 [26] Taiwan | 36 M | Chronic plaque psoriasis for more than 20 y | AZ (1st) | 18 d | Not sure due to retrospective collection | Psoriatic lesions on his lower extremities, which rapidly spread to his trunk and upper extremities within days (PASI 6.5, BSA 5.5% at wk 3) | NR | ADM 40 mg Q2W | ADM 40 mg QW, CsA 200 mg QD | NR | NR |
| Taiwan | 50 F | Chronic plaque psoriasis for 10 y | AZ (NR) | 7 d | Not sure due to retrospective collection | Psoriatic lesions on the trunk and extremities (PASI 5.3, BSA 2% at wk 3) | NR | IXE 80 mg Q4W | IXE 80 mg Q4W | NR | NR |
| Fang et al. 2021 [59] Taiwan | 34 F | PV (PASI 0 without systemic therapy over the past 5 mo) | AZ (1st) | 7 d | NR | An erythematous scaly plaque over the injection site. Scattered psoriatic papules and plaques over trunk and extremities | NR | Intermittent doses of UST and CsA | TCS | Resolved (4 wk) | NR |
| Krajewski et al. 2021 [40] Poland | 46 M | Plaque psoriasis for 24 y (PASI 0 during the last 21 mo) | Pfizer (2nd) | 5 d | NR | Highly inflammatory, psoriatic plaques with gross, silver scaling localized mostly over lower legs. Multiple, smaller lesions were visible over back and chest (PASI 18.5) | NR | DEU in the clinical trial (6 mg orally QD) | NR | NR | None |
| Mieczkowska et al. 2021 [48] USA | 65 M | HCC treated with NIV and poorly controlled psoriasis since childhood | Pfizer (1st) | 7 d | Denied any possible triggers, including changes in med, discontinuation of psoriasis tx, or symptoms of and known exposure to SARS-CoV-2 | Significantly worse pruritus and scattered plaques that had increased in BSA, erythema, and scale | NR | APR, TCS/TCAL | NR | NR | No flare after the 2nd dose |
| Niebel et al. 2021 [46] Germany | 62 M | PV for 40 y, larynx carcinoma in full remission | Pfizer (2nd) | 20 d | NR | Generalized erythemato-squamous plaques (PASI 23 at 4 mo) | NR | Topical tx | Cignoline, TCS, UVB 311 nm, TIL | Improved (NR) | NR |
| Sotiriou et al. 2021 [10] Greece |
49–83 ya M (n = 5) F (n = 8) |
Psoriasis |
AZ (1st) (n = 2) AZ (2nd) (n = 4) Moderna (2nd) (n = 1) Pfizer (2nd) (n = 6) |
5–32 da | NR |
Nail involvement (n = 6) PASI 5.4–16.8a |
NR | NR | GUS (n = 1), IXE (n = 1), RZB (n = 1), APR (n = 1), CsA (n = 1), TCS/TCAL (n = 5), PUVA (n = 2), NBUVB (n = 1) | NR | NR |
| Burlando et al. 2022 [51] Italy | 56 M | Plaque psoriasis | Pfizer (2nd) | 16 d | NR | Psoriasis plaques on the trunk and limbs | NR | NR | NR | NR | None |
| Durmaz et al. 2022 [39] Turkey | 64 M | PV (in remission for more than 1 y), epilepsy under sodium valproate for 30 y | Pfizer (3rd) | 6 wk | No history of any other disease, infection, or additional drug use | Sharply demarcated, erythematous, silver-colored scaly plaques on the bilateral dorsum of the hand, elbow and leg extensor surfaces, and intergluteal region (PASI 4.4) | NR | Without tx | NR | NR | None |
| Kabbani et al. 2022 [52] Belgium | 53 M | HTN, T2DM, thromboembolism, COPD, pulmonary infections, severe plaque psoriasis since 5 y | Pfizer (both) | 1 wk (both) | NR | A mild flare-up of psoriasis over legs after the first dose. Generalized erythematous desquamative plaques and thoracic zona after the second dose | NR | ADM, SEC, GUS, APR, CsA, MTX, TCS, PT | CsA, OAC, OCS, TCS | Improved (NR) | Flare after both doses of Pfizer |
| Koumaki et al. 2022 [38] Greece |
34–67 ya M (n = 3) F (n = 7) |
PsA (n = 4), HTN (n = 2), T2DM (n = 1), dyslipidemia (n = 2), ACS (n = 1), Hashimoto disease (n = 1), celiac disease (n = 1), Crohn's disease (n = 1), COPD (n = 1), arthralgia (n = 1) |
AZ (1st) (n = 1) Pfizer (1st) (n = 1) Pfizer (2nd) (n = 6) Pfizer (both) (n = 2) |
2–25 d | NR | BSA 14%–50%a | NR | ADM (n = 1), SEC (n = 3), UST (n = 1), OCS (n = 1), TCS (n = 2), TCS/TCAL (n=2) | ADM (n = 1), SEC (n = 3), UST (n = 1), APR (n = 1), IVCS (n = 1), OCS (n = 1), TCS (n = 6), TCAL (n = 3) |
Improved (n = 10) 10 d–4 moa |
Flare after both doses of Pfizer (n = 2) None (n = 6) NR (n = 2) |
| Megna et al. 2022 [12] Italy |
39–70 ya M (n = 7) F (n = 3) |
Psoriasis |
AZ (1st) (n = 1) AZ (2nd) (n = 2) Moderna (2nd) (n = 1) Pfizer (1st) (n = 1) Pfizer (2nd) (n = 5) |
5–14 da | NR | PASI 4.3–17.3a | NR | ADM (n = 1), ETN (n = 1), GUS (n = 1), SEC (n = 2), NBUVB (n = 1), without tx (n = 3), topical tx (n = 1) | ADM (n = 2), BRO (n = 1), GUS (n = 1), IXE (n = 2), RZB (n = 1), SEC (n = 2), MTX (n = 1) | NR | NR |
| Pesqué et al. 2022 [28] Spain | 30 F | Mild plaque psoriasis since 2018 | Moderna (1st) | 10 d | Denied any drugs, previous infections, or stressors | Flare of her previous condition with scaly, desquamative plaques primarily over left arm (injection site), and to a lesser extent on the right arm (PASI 2.1, BSA 2.5%) | Normal BR | Topical tx | TCS/TCAL | Resolved (NR) | No flare after the 2nd dose |
| Wei et al. 2022 [9] USA | 56 pt | Psoriasis | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Guttate | |||||||||||
| Yu Phuan et al. 2021 [8] Singapore | 80 F | Stable quiescent psoriasis | Pfizer (3rd) | 7 d | NR | BSA 5% | NR | CsA 25 mg QD | CsA 75 mg QD, TCS | NR | None |
| Sotiriou et al. 2021 [10] Greece | 61 F | Psoriasis | AZ (2nd) | 3 d | NR | PASI 5.9 | NR | NR | NBUVB | NR | None |
| Megna et al. 2022 [12] Italy | 47 M | Psoriasis | Pfizer (2nd) | 9 d | NR | PASI 4.3 | NR | IXE | IXE, TCS/TCAL, PT | NR | None |
| Wei et al. 2022 [9] USA | 1 pt | Psoriasis | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Plaque + Guttate | |||||||||||
|
Huang and Tsai 2021 [50] Taiwan (12 plaque patients, 3 guttate patients) |
Mean age 53.6 y M (n = 8) F (n = 7) |
Plaque psoriasis (n = 15), psoriatic arthritis (n = 7), family history of psoriasis (n = 6), history of erythroderma (n = 3), HTN (n = 5), T2DM (n = 2), CVD (n = 1), HCV infection (n = 1) |
AZ (1st) (n = 3) AZ (2nd) (n = 4) AZ (both) (n = 1) Moderna (1st) (n = 1) Moderna (2nd) (n = 6) |
Mean 9.3 d | No specific aggravating factors, such as upper respiratory infection, excess ultraviolet exposure, change of medications, or psychological stress in all patients |
Mean (SD) PASI 8.0 BSA 8.0% |
NR | GUS (n = 3), IXE (n = 2), RZB (n = 2), ETN (n = 1), ADM (n = 1), ADM + MTX (n = 2), SEC (n = 1), MTX (n = 1), TCS (n = 1) | NR | Improved (mean 64.6 d) (n = 11) |
No flare after the 2nd dose (n = 4) Flare after both doses of AZ (n = 1) None (n = 10) |
| Pustular | |||||||||||
| GPP | |||||||||||
| Chao and Tsai 2021 [26] Taiwan | 40 F | GPP (HMZ IL36RN mutation) since 2 y/o | AZ (NR) | 1 d | Previous triggers included upper respiratory infection, trauma, and gestation. Not sure due to retrospective collection | Fever up to 39 °C, malaise, and new-onset painful rash. Her symptoms recurred twice, each lasting several days, in the following 3 wk | NR | BRO 210 mg Q2W, OAC 20 mg QD | BRO 210 mg Q2W, OAC 50 mg QD | NR | NR |
| Onsun et al. 2021 [60] Turkey | 72 M | Plaque psoriasis, recent history of AKI and HTN | Sinovac (NR) | 4 d | NR | Diffuse erythema, desquamation, and coalescing pustules over the entire body | Elevated levels of acute-phase reactants, COVID-19 PCR(−), PBS(−), normal tumor marker levels | Topical tx | OAC 25 mg QD, IV INX 5 mg/kg | Resolved (NR) | NR |
| Perna et al. 2021 [11] USA | 40s, M | HTN, morbid obesity, depression/anxiety, and psoriasis | Pfizer (1st) | 5 d | No recent illnesses or new med exposures | Tender, erythematous patches and plaques over abdomen and arms, with progression to legs and buttocks. Erythematous patches studded with fine pustules involving trunk, arms, and legs | Leukocytosis and AKI, blood culture(−) | Emollients | IVCS, OCS, antibiotic, antifungal, INX 5 mg/kg (1 dose), CsA 4 mg/kg daily (3 d), taper to 2 mg/kg QD (2 d), SEC | Resolved (NR) | NR |
| Yatsuzuka et al. 2021 [42] Japan | 65 M | HTN and GPP | Pfizer (2nd) | 12 d | No past vaccination-related reaction, recent med changes, or recent infection | Erythematous patches and plaques began to develop on the right cubital fossa, and on the outside of the left upper arm. The eruptions gradually spread to the trunk and inguinal region, and some pustules formed over 1 wk | Leukocytosis and neutrophilia, elevated CRP (5.60 mg/dL) on admin day. Elevated CRP (13.23 mg/dL), hypoalbuminemia (2.6 g/dL), elevated Cr (1.65 mg/dL) on the day after admin | IV INX 5 mg/kg Q8W for 9 y | SEC 300 mg QW until wk 4 | Improved (3 wk) | None |
| Durmaz et al. 2022 [39] Turkey | 25 F | Pustular psoriasis for 11 y (in remission for the last 6 mo) | Pfizer (1st) | 3 d | No previous history of infection, medication, or stress | Non-follicular pustules and local desquamation on erythematous plaques on the anterior-posterior aspect of the trunk, both arms, and proximal thighs | NR | Without tx | NR | NR | NR |
| Frioui et al. 2022 [54] Tunisia | 20 M | Mild plaque psoriasis | Pfizer (1st) | 4 d | No history of new meds or recent illness | Coalescing pustules overlying painful, erythematous skin with yellow crusts on the limbs and desquamation on the trunk. No mucosal membrane or palmoplantar involvement (Naranjo 6) | Neutrophilic leukocytosis, normal serum calcium, and elevated CRP levels, bacterial skin swab(−), COVID-19 PCR(−) | TCS | OAC 25 mg QD, TCS | Resolved (2 wk) | NR |
| Pavia et al. 2022 [56] Italy | 47 F | Plaque psoriasis since 2001 | Pfizer (2nd) | 10 d | Skipped the scheduled administration of UST during the month of vaccine injection | Wide erythematous plaques confluent to both the trunk and the four limbs, covered by large scales (PASI 29.8, BSA > 30%) | Leukocytosis, elevated CRP (14.56 mg/dL), blood culture(−), tumor marker(-) | UST 90 mg since 2016, INF | SC RZB 75 mg/fl (at day 0, wk 2), daptomycin 850 mg QD | Resolved (4 mo) | None |
| Rouai et al. 2022 [57] Tunisia | 66 M | 20-y history of psoriasis, obesity, PsA | Pfizer (NR) | 4 d | No med intake history | Multiple erythematous and scaly plaques with an active border composed of non-follicular pinhead-sized pustules and desquamation, affecting the trunk and the limbs | Neutrophilic leukocytosis, COVID-19 PCR(−) | NR | TCS | Resolved (2 wk) | NR |
| Severe pustular | |||||||||||
| Koumaki et al. 2022 [38] Greece | 53 F | Crohn’s disease, arthralgia, inverse psoriasis | Pfizer (1st) | 20 d | NR | BSA 10% | NR | ADM 4 mg Q2W | IVCS, TCS, OAH | Improved (3 mo) | NR |
| Greece | 61 F | RA, hypothyroidi sm, inverse psoriasis | Pfizer (2nd) | 4 d | NR | BSA 40% | NR | SC MTX 15 mg QW |
SC MTX 15 mg QW, IV INX 5 mg/kg (at d 0, wk 2/6/8) IVCS, OAH |
Improved (2 mo) | None |
| PPP | |||||||||||
| Durmaz et al. 2022 [39] Turkey | 64 M | PPP | Pfizer (2nd) | 7 d | Not receiving any treatment | Sharply circumscribed silver-colored scaly plaques in the hypothenar area of both palmar regions and sharply circumscribed hyperkeratotic plaques and fissures in the plantar region | NR | Without tx | NR | NR | None |
| Piccolo et al. 2022 [45] Italy | 57 M | Moderate plaque psoriasis | Pfizer (NR) | 1 mo | NR | NR | NR | Topical tx | OAC | NR | NR |
| 63 F | Moderate plaque psoriasis | Pfizer (NR) | 1 mo | NR | NR | NR | Topical tx | OAC | NR | NR | |
| Erythrodermic | |||||||||||
| Durmus et al. 2022 [61] Turkey | 42 M | Plaque psoriasis and PsA for 20 y | Pfizer (1st) | 4 wk | Mildly symptomatic COVID-19 infection 8 mo ago treated with oral favipiravir for 5 d with complete improvement | Widespread, near total-body erythema with desquamation, along with scaly erythematous plaques on the extremities. Severe palmoplantar hyperkeratosis and fissuring, along with mild erythematous silvery-white scaly patches on the scalp. Pitting and onycholysis over all fingernails (PASI 48.6, BSA 95%) | Leukocytosis with neutrophilia, and elevated CRP levels, normal serum alb and TP levels | OAC, MTX, ADM without improvement, started SEC 2y ago | OCS, IXE | Resolved (3 wk) | NR |
| Lopez et al. 2022 [55] New Mexico | 58 M | Psoriasis since 2019, HTN, IV drug use with heroin, osteomyelitis, untreated hepatitis C, tobacco use disorder | Pfizer (2nd) | 4 d | NR | Large, raised, erythematous areas and silver scaling involving the bilateral lower extremities, abdomen, chest, back, bilateral upper extremities, and face (BSA > 80%) | Normal BR, Hepatitis C vaccine genotype 1a(+), HIV(−),serum pt EP(−), urine pt EP(−), Sezary panel(−) | Coal tar, Echinacea lotion | TCS, emollients, OAH | Improved (6 d) | None |
| Nia et al. 2022 [53] USA | 58 M | Guttate psoriasis with occasional flares | Pfizer (1st) | 1 d | No new medications or changes to drugs, changes in diet, or contact with a possible allergen or irritant | Confluent bright erythema with a thick and loose yellow-gray scale on the face, torso, and upper extremities. Annular erythematous plaques coalesced into confluent plaques with hyperkeratotic scales on the lower extremities and buttocks | A mild leukocytosis with mature granulocytosis and mild thrombocytosis. Elevated Cr level with AKI. Normal LFT and electrolytes. COVID-19 PCR(−), blood culture(−), HAV(−), HBV(−), HCV(−), HIV(−), syphilis(−) | TCS | CsA 3 mg/kg QD, UVB 3 times/wk for 3 mo, TCS, OAC | Resolved (3 mo) | NR |
| Tran et al. 2022 [58] Vietnam | 30 F | 15-y history of chronic plaque psoriasis (PASI < 10) | Pfizer (2nd) | 7 d | Pause all anti-psoriatic treatments due to fear of adverse drug–vaccine interactions after vaccination | Fatigue, fever, malaise, and poor oral intake lasting for several days. Generalized erythematous patches with marked desquamation (BSA > 75%) | Normal BR except severe hypocalcemia and a mildly elevated eosinophil count | SEC, traditional herbal remedies, TCS, Vit D | OAC 25 mg QD | Improved (2 wk) | None |
| Vietnam | 45 F | Moderate stable plaque psoriasis for more than 20 y (PASI 10) | Pfizer (2nd) | 7 d | Denied using any systemic meds | Diffusely erythematous and scaly, marked edema of lower extremities (BSA 90%) | Normal BR | TCS/TCAL | NR | NR | None |
| PsA | |||||||||||
| Spinelli et al. 2022 [43] Italy | 47 F | PsA | mRNA vaccine (2nd) | 18 d | NR | III PIP arthritis | NR | Without tx | None | Resolved (10 d) | None |
| 58 F | PsA | mRNA vaccine (2nd) | 3 d | NR | Inflammatory back and neck pain | NR | TNF-αi | NSAID (once) | Resolved (3 d) | None | |
| PsA + PPP | |||||||||||
|
Quattrini et al. 2021 [44] Italy (PsA + PPP) |
83 F | PPP since 1996, HTN, T2DM under med | Pfizer (2nd) | 2 d | NR | Both hands on palmar side showed psoriasis with erythematous, scaly plaques, while on dorsum and wrist joints, painful edema was present. Dactylitis was also detected in all fingers associated with severe functional impairment | NR | MTX 10 mg Q10D | OCS, increased MTX dosage to 10 mg QW | Improved (NR) | None |
| Not specified | |||||||||||
| McMahon et al. 2021 [13] USA | 2 pt | Psoriasis | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Nagrani et al. 2021 [5] India | 56 F | Psoriasis in remission for 6 mo | AZ (both) | 2 d | NR | Exacerbation of psoriasis lesions | NR | Emollients | APR (10 mg on day 1, 30 mg BID by day 7), OAH, emollients | Improved (7 d) | Flare after both doses of AZ |
| Wang et al. 2021 [62] China | 114 pt | Psoriasis | NR | NR | NR | NR | NR | Nonadherence to treatment (n = 31), TNF-ai (n = 11), IL-17i (n = 15) | NR | NR | NR |
| Català et al. 2022 [37] Spain | 6 pt | Psoriasis | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Mahil et al. 2022 [49] UK |
Age NR M (n = 1) F (n = 7) |
Psoriasis | Pfizer (2nd) | NR | NR | NR | NR | TNF-αi (n = 4), IL-17i (n = 1), IL-23i (n = 1), MTX (n = 2) | NR | NR | None |
| Musumeci et al. 2022 [47] Italy | 1 pt | Plaque psoriasis | Pfizer (NR) | NR | NR | NR | NR | INX | NR | NR | NR |
| Wei et al. 2022 [9] USA |
27–76 ya M (n = 3) F (n = 3) |
Psoriasis |
Moderna (both) (n = 1) Moderna (2nd) (n = 4) Pfizer (2nd) (n = 1) |
6–90 d | Previous COVID-19 infection (n = 5) | BSA range from < 10 to 60% | NR | NR | TIL (n = 2), RZB (n = 2), APR (n = 2), NBUVB PT (n = 1), TCS (n = 3) |
Improved (n = 4) Resolved (n = 1) Unknown (n = 1) |
Flare 7 d after both doses (n = 1) None (n = 5) |
ACS acute coronary syndrome, ADM adalimumab, admin admission, AKI acute kidney injury, APR apremilast, AZ AstraZeneca, BR blood routine, BRO brodalumab, BSA body surface area, COPD chronic obstructive pulmonary disease, COVID-19 coronavirus disease, Cr creatinine, CRP C-reactive protein, CsA cyclosporine, CVD cardiovascular disease, d day, DEU deucravacitinib, EP electrophoresis, ETN etanercept, GPP generalized pustular psoriasis, GUS guselkumab, HAV hepatitis A virus, HBV hepatitis B virus, HCC hepatocellular carcinoma, HCV hepatitis C virus, HMZ homozygous, HTN hypertension, IL-17i interleukin-17 inhibitors, IL-23i interleukin-23 inhibitors, IL36RN interleukin 36 receptor antagonist, INF interferon, INX infliximab, IV intravenous, IVCS intravenous corticosteroids, IXE ixekizumab, LFT liver function test, mo month, mRNA messenger ribonucleic acid, MTX methotrexate, NBUVB narrow band ultraviolet B, NIV nivolumab, NR not reported, NSAID non-steroidal anti-inflammatory drug, OAC oral acitretin, OCS oral corticosteroids, PASI Psoriasis Area and Severity Index, PBS peripheral blood smear, PCR polymerase chain reaction, PCT procalcitonin, PIP proximal interphalangeal, PPP palmoplantar pustulosis psoriasis, PsA psoriatic arthritis, pt patients(s), PT phototherapy, PUVA psoralen and UVA, PV psoriasis vulgaris, Q10D every 10 days, Q2W every 2 weeks, Q4W every 4 weeks, Q8W every 8 weeks, QD every day, QW every week, RA rheumatoid arthritis, RZB risankizumab, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, SC subcutaneous, SEC secukinumab, T2DM type 2 diabetes mellitus, TCAL topical calcipotriol, TCS topical corticosteroids, TIL tildrakizumab, TNF-αi TNF-alpha inhibitors, TP total protein, Tx treatment, UST ustekinumab, UVB ultraviolet B, Vit vitamin, wk week, y year, y/o year old
aRange
Patient Characteristics
Detailed information on the included patients is presented in Tables 1 and 2. The characteristics of the included studies are summarized in Table 3. The new-onset group consisted of 35 patients, mostly from America, ranging from 23 to 79 years in age. Most studies did not document patient sex, but among those that did, women slightly outnumbered men. The majority of patients presented with plaque psoriasis (17 patients), followed by guttate psoriasis (10 patients), and generalized pustular psoriasis (GPP) and nail psoriasis were reported in two patients and one patient, respectively.
Table 3.
Summary of characteristics of the included studies
| Psoriasis type | Study (n) | Patient (n) | Country, n (%) | Age, ya | Sex, n (%) | Vaccine, n (%) | Dose, n (%) | Onseta | Outcome, n (%) | Time to improvement/resolutiona | Further vaccine, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| New onset | |||||||||||
| Plaque | 2 | 17 | America 17 (100) | 67 | NR 17 (100) |
Moderna 1 (5.88) NR 16 (94.12) |
NR 17 (100) | NR | NR 17 (100) | NR | NR |
| Guttate | 5 | 10 |
Asia 1 (10.00) America 7 (70.00) Europe 2 (20.00) |
23–79 |
M 2 (20.00) F 2 (20.00) NR 6 (60.00) |
Moderna 1 (10.00) Pfizer 3 (30.00) NR 6 (60.00) |
1st 2 (20.00) 2nd 2 (20.00) NR 6 (60.00) |
2–14 d |
Improved 2 (20.00) Resolved 2 (20.00) NR 6 (60.00) |
2 wk |
Flare (2nd) 2 (20.00) None 2 (20.00) NR 6 (60.00) |
| GPP | 2 | 2 | Europe 2 (100) | 64–66 | F 2 (100) |
AZ 1 (50.00) Pfizer 1 (50.00) |
1st 2 (100) | 21 d |
Improved 1 (50.00) Resolved 1 (50.00) |
1 mo |
Flare (2nd) 1 (50.00) No flare (2nd) 1 (50.00) |
| Nail psoriasis | 1 | 1 | America 1 (100) | 76 | F 1 (100) | Pfizer 1 (100) | 2nd 1 (100) | 7 d | Improved 1 (100) | 2 mo | None 1 (100) |
| Not specified | 3 | 5 |
Asia 1 (20.00) America 1 (20.00) Europe 3 (60.00) |
65 |
F 1 (20.00) NR 4 (80.00) |
AZ 1 (20.00) NR 4 (80.00) |
2nd 1 (20.00) NR 4 (80.00) |
10 d |
Improved 1 (20.00) NR 4 (80.00) |
7 d |
None 2 (40.00) NR 3 (60.00) |
| Total | 11 | 35 |
Asia 2 (5.71) America 26 (74.29) Europe 7 (20.00) |
23–79 |
M 2 (5.71) F 6 (17.14) NR 27 (77.14) |
AZ 2 (5.71) Moderna 2 (5.71) Pfizer 5 (14.29) NR 26 (74.29) |
1st 4 (11.42) 2nd 4 (11.42) NR 27 (77.14) |
2–21 d |
Improved 5 (14.29) Resolved 3 (8.57) NR 27 (77.14) |
7 d–2 mo |
No flare (2nd) 1 (2.86) Flare (2nd) 3 (8.57) None 5 (14.29) NR 26 (74.29) |
| Flare | |||||||||||
| Plaque | 14 | 101 |
Asia 6 (5.00) America 57 (56.44) Europe 38 (37.62) |
30–70 |
M 24 (23.76) F 21 (20.79) NR 56 (55.45) |
AZ 13 (12.87) Moderna 3 (2.97) Sinovac 1 (1.00) Pfizer 28 (27.72) NR 56 (55.45) |
1st 10 (9.90) 2nd 30 (29.70) 3rd 1 (1.00) Both 3 (2.97) NR 57 (56.44) |
2 d–6 wk |
Improved 11 (10.89) Resolved 3 (29.70) NR 87 (86.14) |
10 d–4 mo |
No flare (2nd) 2 (1.98) Flare (both) 4 (3.96) None 10 (9.90) NR 85 (84.16) |
| Guttate | 4 | 4 |
Asia 1 (25.00) America 1 (25.00) Europe 2 (50.00) |
47–80 |
M 1 (25.00) F 2 (50.00) NR 1 (25.00) |
AZ 1 (25.00) Pfizer 2 (50.00) NR 1 (25.00) |
2nd 1 (25.00) 3rd 2 (50.00) NR 1 (25.00) |
3 d–9 d | NR 4 (100) | NR |
None 3 (75.00) NR 1 (25.00) |
| Plaque + guttate (12 plaque, 3 guttate) | 1 | 15 | Asia 15 (100) | Mean 53.6 |
M 8 (53.33) F 7 (46.67) |
AZ 4 (26.67) Moderna 11 (73.33) |
1st 4 (26.67) 2nd 11 (73.33) |
Mean 9.3 d | NR 15 (100) | NR |
No flare (2nd) 4 (26.67) Flare (both) 1 (6.67) None 10 (66.67) |
| Pustular | 11 | 13 |
Asia 5 (38.46) Africa 2 (15.38) America 1 (7.69) Europe 5 (38.46) |
20–72 |
M 8 (61.54) F 5 (38.46) |
AZ 1 (7.69) Sinovac 1 (7.69) Pfizer 11 (84.62) |
1st 4 (30.77) 2nd 4 (30.77) NR 5 (38.46) |
1 d–1 mo |
Improved 3 (23.08) Resolved 5 (38.46) NR 5 (38.46) |
2 wk–4 mo |
None 4 (30.77) NR 9 (69.23) |
| PsA | 1 | 2 | Europe 2 (100) | 47–58 | F 2 (100) | mRNA vaccine 2 (100) | 2nd 2 (100) | 3 d–18 d | Resolved 2 (100) | 3 d–10 d | None 2 (100) |
| Erythrodermic | 4 | 5 |
Asia 3 (60.00) America 2 (40.00) |
30–58 |
M 3 (60.00) F 2 (40.00) |
Pfizer 5 (100) |
1st 2 (40.00) 2nd 3 (60.00) |
1 d–4 wk |
Improved 2 (40.00) Resolved 2 (40.00) NR 1 (20.00) |
6 d–3 mo |
None 3 (60.00) NR 2 (40.00) |
| PsA + PPP | 1 | 1 | Europe 1 (100) | 83 | F 1 (100) | Pfizer 1 (100) | 2nd 1 (100) | 2 d | Improved 1 (100) | NR | None 1 (100) |
| Not specified | 7 | 138 |
Asia 115 (83.33) America 8 (5.80) Europe 15 (10.87) |
27–76 |
M 4 (2.90) F 11 (7.97) NR 123 (89.13) |
AZ 1 (0.75) Moderna 5 (3.62) Pfizer 10 (7.25) NR 122 (88.41) |
2nd 13 (9.42) Both 2 (1.45) NR 123 (89.13) |
2–90 d |
Improved 5 (3.62) Resolved 1 (0.72) Unknown 1 (0.72) NR 131 (94.93) |
7 d |
Flare (both) 2 (1.45) None 13 (9.42) NR 123 (89.13) |
| Total | 36 | 279 |
Asia 145 (51.97) Africa 2 (0.72) America 69 (24.73) Europe 63 (22.58) |
20–83 |
M 48 (17.20) F 51 (18.28) NR 180 (64.52) |
AZ 20 (7.17) Moderna 19 (6.81) Sinovac 2 (0.72) Pfizer 57 (20.43) mRNA vaccine 2 (0.72) NR 179 (64.16) |
1st 20 (7.17) 2nd 65 (23.30) Both 5 (1.79) 3rd 3 (1.08) NR 186 (66.67) |
1–90 d |
Improved 22 (7.88) Resolved 13 (4.66) Unknown 1 (0.36) NR 243 (87.10) |
3 d–4 mo |
No flare (2nd) 6 (2.15) Flare (both) 7 (2.51) None 46 (16.49) NR 220 (78.85) |
AZ AstraZeneca, d day, F female, GPP generalized pustular psoriasis, M male, mRNA messenger ribonucleic acid, NR not reported, PPP palmoplantar pustulosis psoriasis, PsA psoriatic arthritis, w week
aRange
The flare group consisted of 279 patients, ranging from 20 to 84 years in age, who mostly experienced plaque-type psoriasis, followed by pustular, guttate, and erythrodermic psoriasis and PsA. Approximately 52% of patients were from Asian countries, followed by America (25%) and European countries (23%). The youngest patient was 20 years of age and developed pustular psoriasis, but the majority of cases were middle-aged or older patients. Similar to the new-onset group, most studies did not report patient sex, but among those that did, women slightly outnumbered men.
Vaccine Type, Vaccine Dose, and Psoriasis Onset Time After Vaccination
In the new-onset group, 14% of patients were administered the BioNTech/Pfizer vaccine [27, 29, 33–35], 6% were administered the Moderna vaccine [14, 28], and 6% the AstraZeneca vaccine [5, 36], although the vaccine type was not reported for the majority of patients [9, 37]. The vaccine dose was only reported for eight patients [5, 27–29, 33–36], with four new-onset psoriasis cases reported after patients received the second vaccine dose [5, 27, 28, 34], and four cases reported after the first dose [29, 33, 35, 36]. A wide range of onset times was observed, ranging from 2 to 21 days following vaccination [33, 36]. The longest onset time was observed in a patient with new-onset GPP [36], with the majority of new-onset psoriasis cases presenting 1–2 weeks following vaccination.
In the flare group, most patients received mRNA vaccines (28%) [8, 10–12, 28, 38–58], with the BioNTech/Pfizer vaccine (20%) reported more often than the Moderna vaccine (7%), followed by the AstraZeneca vaccine (7%) [5, 10, 12, 26, 38, 50, 59] and the Sinovac vaccine (1%) [41, 60]. Flares were most commonly reported following the second vaccine dose (23%) [5, 10, 12, 38–44, 49–51, 55, 56, 58], followed by the first vaccine dose (7%) [10–12, 26, 28, 38, 39, 48, 50, 53, 54, 59], both the first and second doses (2%) [38, 52], and the third dose (1%) [8, 39]. The onset time in the flare group ranged from 1 day to 90 days. The shortest onset times were reported for a 40-year-old man who experienced a GPP flare following the AstraZeneca vaccine and a 58-year-old woman with erythrodermic psoriasis following the first dose of the BioNTech/Pfizer vaccine [26, 53]. The longest onset time was reported for a 27-year-old woman who experienced a psoriasis flare after the second dose of the BioNTech/Pfizer vaccine [9].
History of COVID-19 Infections or Potential Non-Vaccine Triggers
Most patients denied the possibility of other non-vaccine triggers in both the new-onset and flare groups. However, previous COVID-19 infections were identified in six patients who experienced psoriasis flares after receiving the Moderna and BioNTech/Pfizer vaccines [9, 61]. Similarly, in one patient with new-onset nail psoriasis, a history of asymptomatic SARS-CoV-2 infection 6 months prior to vaccination was reported [27]. One patient who experienced a GPP flare after receiving the AstraZeneca vaccine had a history of experiencing psoriasis flares, with prior triggers including upper respiratory infection, trauma, and pregnancy [26]. An association between COVID-19 vaccination and psoriasis flares was highly suspected, but the study was limited by its retrospective design, and a causal relationship could not be determined [26].
Skin Manifestations, and Laboratory Studies
Typical psoriatic skin manifestations were reported for each psoriasis subtype in both the new-onset and flare groups. Only three studies documented the severity of new-onset psoriasis [5, 9, 27]. A patient who experienced new-onset nail psoriasis was reported by Ricardo and Lipner, characterized by nailfold erythema and degenerative changes in the small joints of the hands after receiving the second BioNTech/Pfizer vaccine dose, with a nail psoriasis severity index (NAPSI) of 18 points [27]. Two cases of new-onset psoriasis involving body surface areas (BSAs) of 30% and 60% were reported after the second AstraZeneca and Moderna vaccine doses, respectively [5, 9]. The severity of psoriasis flares was reported by 15 studies according to either PASI or BSA [8–10, 12, 26, 28, 40, 41, 46, 50, 55, 58, 61], ranging from PASI 2.1 to 48.6 and BSA 2–95%.
In the new-onset group, laboratory studies were only reported in three cases, including two cases with guttate psoriasis and one case with GPP [28, 33, 36]. In the two cases with guttate psoriasis, one patient presented with an elevated C-reactive protein (CRP) level [28], whereas the other patient was reported to have a CRP level and an erythrocyte sedimentation rate (ESR) within the normal limits [33]. No patients presented with elevated anti-streptolysin O (ASLO) titers, and a throat culture performed in the case reported by Pesqué et al. [28] was negative. Twelve studies including 14 patients in the flare group documented laboratory findings [11, 28, 41, 42, 53–58, 60, 61]. A slightly elevated CRP level was reported for a 51-year-old man who experienced a plaque psoriasis flare after receiving the second dose of the BioNTech/Pfizer vaccine [41]. Leukocytosis and elevated levels of acute-phase reactants were individually reported in six cases with GPP [11, 42, 54, 56, 57, 60]. A 65-year-old man who developed severe GPP following the second dose of the BioNTech/Pfizer vaccine, reported by Yatsuzuka et al., presented with a high CRP level, hypoalbuminemia, and an elevated creatinine level on the day after admission [42]. Durmus et al. reported a 42-year-old man who developed psoriatic erythroderma with leukocytosis, neutrophilia and an elevated CRP level, which was gradually resolved with oral prednisolone and ixekizumab treatment [61]. Mild leukocytosis with mature granulocytosis and mild thrombocytosis, together with an elevated creatinine level, was described for a case of erythrodermic psoriasis reported by Nia et al., which was successfully resolved following treatment with cyclosporine, UVB phototherapy, and topical corticosteroids [53].
Treatment and Outcomes for New-Onset Psoriasis or Psoriasis Flares
In the new-onset group, most patients were treated using topical agents, including topical corticosteroids, calcipotriol, or emollients. Oral acitretin, administered once daily at 20 mg in a case of GPP and 25 mg once daily in another case of psoriasis without mentioning subtype, were prescribed [9, 36]. Methotrexate, administered once daily at 15 mg, was prescribed in one case with annular pustular psoriasis [35]. An older Indian patient who developed new-onset psoriasis without mentioning subtype following a second dose of the AstraZeneca vaccine was treated with apremilast at 10 mg on day 1, which was titrated to 30 mg twice daily by day 7, and the patient showed improvement on day 7 [5]. Ixekizumab was administered to an 89-year-old man with new-onset psoriasis affecting 60% BSA following a second dose of the Moderna vaccine, and the patient recovered after treatment [9]. Most patients showed improvement or resolution after treatment, and the time to improvement or resolution ranged widely, from 7 days to 2 months. Patients with nail psoriasis required the longest time to improve (2 months), followed by GPP (1 month) and guttate psoriasis (2 weeks). Two patients with new-onset guttate psoriasis following the first dose of the BioNTech/Pfizer vaccine also experienced a psoriasis flare following the second vaccine dose [29, 33]; however, one patient with new-onset GPP following the first dose of the AstraZeneca vaccine did not experience a psoriasis flare after the second dose [36].
In the flare group, various treatments were used to control underlying psoriasis, including topical treatments, such as topical corticosteroids, topical calcipotriol, and emollients; systemic immunomodulatory agents, such as acitretin, cyclosporine, methotrexate, and apremilast; narrow-band UVB phototherapy; and biologics, such as tumor necrosis factor-α (TNF-α) inhibitors (adalimumab, etanercept, infliximab), IL-17 inhibitors (brodalumab, ixekizumab, secukinumab), IL-23 inhibitors (guselkumab, risankizumab), IL-12/23 inhibitors (ustekinumab), and tyrosine kinase 2 (TYK2) inhibitor (deucravacitinib). Treatments resulted in stable psoriasis control or remission in nine cases (3%) [5, 8, 39, 40, 54–57, 59]. However, treatment resulted in only partial control of chronic and relapsing psoriasis in six cases (1%) [26, 41, 47, 53, 58]. To control psoriasis flares following vaccination, dosage titration of prior treatments was documented in three cases (1%) [8, 26, 44]. Six cases (2%) were maintained at the dosage used for prior treatments [26, 28, 38, 43], and 19 patients (7%) were treated with add-on medications, such as topical agents, phototherapy, or systemic therapies, including topical emollients, betamethasone, clobetasol propionate, calcipotriol/betamethasone combination, isoconazole and diflucortolone valerate, systemic methylprednisolone, methotrexate, cyclosporine, apremilast, infliximab, tildrakizumab, and brodalumab [5, 12, 26, 38, 46, 52, 53, 59]. Eighteen cases (6%) changed their treatment regimens completely to achieve disease control [11, 12, 38, 43, 45, 54–58, 60, 61]. Among patients with psoriasis flares who were receiving prior treatment with biological agents, most (43%) were using IL-17 inhibitors [12, 26, 38, 49, 50, 58, 61, 62], followed by TNF-α inhibitors (42%) [12, 26, 38, 43, 47, 49, 50, 62, 63], IL-23 inhibitors (10%) [12, 43, 49, 50, 62], IL-12/23 inhibitors (4%) [38, 56, 59], and TYK2 inhibitor (1%) [40]. Secukinumab [12, 38, 50, 58, 61] and adalimumab [12, 26, 38, 50] were the most frequently used biological agents. Improvement and resolution were achieved for most of the patients, with treatment times ranging from 3 days to 2 months. Six patients (2%) who experienced psoriasis flares following the first vaccine administration did not experience another flare episode after the second vaccine administration [28, 48, 50]. However, psoriasis flares after both vaccine doses were reported in seven patients (3%) [5, 9, 38, 41, 50, 52]. No patients died from new-onset psoriasis or psoriasis flares following COVID-19 vaccination in all included studies.
Discussion
In this study, we systematically reviewed all available literature regarding new-onset psoriasis or psoriasis flares following COVID-19 vaccination. A total of 11 studies, including 35 patients with new-onset psoriasis, and 36 studies, including 279 patients with psoriasis flares, were identified and included in this study. The most common psoriasis subtype observed following vaccination was plaque-type psoriasis. mRNA vaccines, including the Moderna and BioNTech/Pfizer vaccines, were frequently associated with subsequent psoriasis episodes. Psoriasis episodes were reported after the first, second, and third vaccine doses, with most episodes reported following the second dose in the flare group. Delayed onset, ranging from 2 to 21 days, was reported for the new-onset group, whereas onset ranged from 1 to 90 days for the flare group. Most patients experienced favorable outcomes with improvement or resolution occurring from 3 days to 4 months following treatment initiation. The purposes of our study were to include and categorize psoriasis cases following COVID-19 vaccination and to explore the possible association between COVID-19 vaccines and psoriasis development.
COVID-19 vaccination may be a triggering factor for psoriasis, indicated by the short intervals between vaccination and psoriasis onset in the absence of other significant factors. The potential for vaccines to trigger psoriasis has previously been suggested for other vaccines, including influenza, rubella, Bacillus Calmette-Guerin (BCG), tetanus-diphtheria, and pneumococcal polysaccharide vaccines [50, 64–67]. The development of psoriatic skin lesions involves various mechanisms. Koebner phenomenon, a characteristic development associated with psoriatic skin lesions, may occur at the site of skin injury associated with vaccination. IL-6 can be induced by various components, such as mycobacterial heat shock proteins, and the diphtheria toxoid present in BCG, tetanus-diphtheria, and influenza vaccines has been linked to the production of Th17 cells, which can further aggravate psoriasis [65, 66]. In our study, no specific vaccine type was associated with a higher rate of new-onset psoriasis, but a large number of psoriasis flares was found after mRNA vaccines, including the Moderna and BioNTech/Pfizer vaccines. The most commonly administered COVID-19 vaccines rely on adenovirus vectors, mRNA, or virus-related proteins, which can trigger Th1 and Th17 responses [1, 50]. The increased production of TNF-α and type I interferon (IFN-I) by CD4+ T cells and plasmacytoid dendritic cells (pDC) may also play roles in psoriasis activation [68–70]. We found that a large number of patients (23%) experienced psoriasis flares following the second vaccine dose. In addition, three patients in the new-onset group (9%) and seven patients in the flare group (3%) experienced psoriasis episodes after both the first and second vaccine doses. Only one patient in the new-onset group (3%) and six patients in the flare group (2%) experienced psoriasis events after the first vaccine dose but not after the second vaccine dose. However, we could not define any specific factors able to predict the occurrence of a psoriasis event due to a lack of detailed information provided by many articles. Further controlled studies are warranted to unravel the relationship between COVID-19 vaccines and psoriasis, including identifying the potential underlying immunologic reactions.
The COVID-19 pandemic introduced substantial obstacles to the effective management of psoriasis. The safety of administering immunomodulators to psoriatic patients was a major concern during the COVID-19 pandemic [71]. Increasingly available data regarding the response to COVID-19 vaccine administration has led to the overwhelming recommendation that psoriatic patients should undergo vaccination regardless of the use of biological agents because the benefits associated with preventing severe SARS-CoV-2 infection outweigh the potential risk of adverse events [20, 21, 72, 73]. According to a population-based cohort study by Pahalyants et al., immunomodulatory biologics are not associated with COVID-19 infection or subsequent mortality [74]. Several real-world studies have also demonstrated that biologics are safe for the management of patients with moderate to severe psoriasis who have been vaccinated against COVID-19 [22, 75]. As of January 2022, approximately 12 billion vaccine doses have been administered, 140 vaccines have been developed in clinical trials, and a number of vaccines have reached approval for human use [76]. Among all COVID-19 vaccines, BioNTech/Pfizer and Moderna vaccines were first authorized for use in American and European countries, and were initially recommended for people older than 16 years and older than 18 years, respectively. A third COVID-19 vaccine, from AstraZeneca, was then approved in January 2021 for people older than 18 years. The initial indications have been extended for use in children aged > 6 years and > 5 years in the Moderna and BioNTech/Pfizer vaccines, respectively [77, 78]. Outside of the US and European Union, China’s Sinovac was approved for emergency use in China in July 2020. However, major concerns were raised due to the fast-tracked approval processes for Sinovac, and both the public and the scientific community were skeptical about its safety and efficacy [79]. In our study, psoriasis flare was frequently noted following mRNA vaccines, especially the BioNTech/Pfizer vaccine. It is noteworthy that the result could be biased by the number of vaccinations performed with each vaccine, which was possibly influenced by the approval time and countries, the vaccine’s acceptability to the public, and its extended indications to children and young adults. In addition, publication bias might negatively affect the results of the current study. Since the included studies were mostly case reports or case series, selective reporting cases with positive findings and relatively low level of evidence in each report should be noted. Quality assessments ranged from 4 to 7 points for case reports and 8 points (fair) for all observational cohort studies, respectively, but challenge/rechallenge phenomenon and dose–response effect were difficult to assess (Table S2 and Table S3, see ESM). To elucidate the causal relationship between psoriasis and COVID-19 vaccines, more data from population-based analysis and pharmacovigilance databases are warranted in the future study.
In addition to COVID-19 vaccines, COVID-19 infections could also cause psoriasis [80]. Dermatological symptoms during COVID-19 infection have been proposed to be related to angiotensin-converting enzyme (ACE) 2, which shows a strong affinity for the SARS-CoV-2 spike protein [81, 82]. ACE2 hyperactivity in COVID-19 patients has been associated with a higher incidence of cardiovascular events, in addition to the exacerbation of psoriasis [82]. We found past histories of SARS-CoV-2 infection in one patient with new-onset nail psoriasis and six patients with psoriasis flares, all of whom developed psoriasis after receiving mRNA vaccines. Infection status was revealed in some patients, and we noticed that GPP and erythrodermic psoriasis were frequently associated with higher levels of acute-phase reactants as opposed to other types of psoriasis. In our study, despite the use of different therapies to control psoriasis, severe cases with high PASI scores or large BSA involvement were primarily treated with biological agents, including TNF-α inhibitors, IL-17 inhibitors, IL-23 inhibitors, IL-12/23 inhibitors, and TYK2 inhibitors. Generally, GPP, severe pustular psoriasis, erythrodermic psoriasis, and psoriasis with larger skin involvement require the use of one or more systemic treatments and require longer to recover than plaque or guttate psoriasis. Although the avoidance of systemic corticosteroids is typically recommended in the treatment of psoriasis vulgaris due to the possibility of rebound phenomenon, a recent meta-analysis questioned this commonly propagated belief and found low rates of rebound flares [83]. Generally, a gradual tapering of systemic corticosteroids was recommended for psoriatic patients [84]. In our study, systemic corticosteroids were administered in eight cases during the acute phase of psoriasis (including plaque, palmoplantar pustulosis, severe pustular, generalized pustular, and erythrodermic psoriasis and PsA) [11, 38, 44, 52, 61]. Intravenous methylprednisolone administered daily at 40–60 mg and oral prednisolone administered daily at 25–60 mg, in addition to other systemic agents, were used for disease control. All cases were successfully treated, with improvement and resolution occurring within 4 months and with no reported mortality.
This study had some limitations. First, although most of the included studies were case reports with detailed documentation of patients’ clinical conditions, some of the studies were observational studies or data from retrospective database collection that lacked detailed information. Some of the results, including vaccine doses, other triggers, laboratory studies, treatments, and disease outcomes, were not reported by all studies, which prevented the determination of any causal relationships between COVID-19 vaccines and psoriasis. Second, severity scores, such as the PASI score, NAPSI score, and BSA scale, are important parameters for evaluating disease severity in psoriasis patients; however, severity assessments were only reported in a limited number of studies. Detailed documentation of psoriasis disease severity at pre-vaccination, post-vaccination, and post-treatment time points is warranted in further studies. Third, a limited number of cases reported whether the patient continued to receive vaccine doses in cases in which psoriasis developed after the first dose, and reactions to later vaccines were rarely reported. Omicron, the new SARS-CoV-2 variant, has recently spread worldwide, and the necessity of a third vaccine dose has been widely discussed [85, 86]. Various governments have promoted the administration of a third booster vaccine dose to achieve better coverage and protect against Omicron-mediated COVID-19 infection [87]. In the current study, only three cases with psoriasis flares were reported following a third vaccine dose in the included studies. Long-term follow-up of all patients who develop psoriasis after vaccination and detailed documentation of CAEs after the third COVID-19 vaccine dose are crucial for exploring the relationship between COVID-19 vaccines and psoriasis onset.
Conclusions
Both new-onset psoriasis and psoriasis flares were reported as potential CAEs following COVID-19 vaccination. Psoriatic patients may require regular follow-up before and after COVID-19 vaccination. More investigations remain necessary to elucidate the association between COVID-19 vaccines and psoriasis and the underlying mechanisms that drive the development of psoriasis following vaccination.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
This study was supported by research grants from the Ministry of Science and Technology, Taiwan (Grant No. MOST 108-2314-B-182A-006-MY3, 108-2320-B-182A-023-MY3, 109-2320-B-182A-008-MY3, 110-2320-B-182A-014-MY3, 108-2314-B-182A-104-MY3, 110-2326-B-182A-003-, 111-2314-B-182A-111-MY3, 111-2314-B-182A-113-MY3) and Chang Gung Memorial Hospital, Taiwan (grant no. CMRPG2J0221-2, CMRPG3L0491, CMRPG3I0381-2, CIRPG3I0024-5, CIRPG3I0044-5, CIRPG2I0014-5, CORPG3L0471).
Conflict of interest
None declared.
Availability of data and material
Chun-Bing Chen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author contributions
Conceptualization, P.C.W. and C.B.C.; methodology, I.H.H. and C.W.C.; software and formal analysis, P.C.W.; investigation and resources, C.C.T., W.H.C. and C.B.C.; data curation, C.C.T., W.H.C. and C.B.C.; writing—original draft preparation, P.C.W., I.H.H., C.B.C.; writing—review and editing, W.H.C. and C.B.C.; visualization, P.C.W. and C.W.C.; supervision, W.H.C. and C.B.C.; funding acquisition, W.H.C. and C.B.C. All authors have read and agreed to the published version of the manuscript.
Statement on any prior presentation
The content of this manuscript has not been published entirely or in part, is not under consideration by another journal, and will not be simultaneously submitted elsewhere.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
References
- 1.Niebel D, Novak N, Wilhelmi J, Ziob J, Wilsmann-Theis D, Bieber T, et al. Cutaneous adverse reactions to COVID-19 vaccines: insights from an immuno-dermatological perspective. Vaccines (Basel). 2021;9(9):944. doi: 10.3390/vaccines9090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritchie H, Mathieu E, Rodés-Guirao L, Appel C, Giattino C, Ortiz-Ospina E, et al. Coronavirus Pandemic (COVID-19). OurWorldInData.org; 2020. https://ourworldindata.org/coronavirus.
- 3.Bellinato F, Maurelli M, Gisondi P, Girolomoni G. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10(22):5344. doi: 10.3390/jcm10225344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun Q, Fathy R, McMahon DE, Freeman EE. COVID-19 vaccines and the skin: the landscape of cutaneous vaccine reactions worldwide. Dermatol Clin. 2021;39(4):653–673. doi: 10.1016/j.det.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagrani P, Jindal R, Goyal D. Onset/flare of psoriasis following the ChAdOx1 nCoV-19 Corona virus vaccine (Oxford-AstraZeneca/Covishield): report of two cases. Dermatol Ther. 2021;34(5):e15085. doi: 10.1111/dth.15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedrazini MC, da Silva MH. Pityriasis rosea-like cutaneous eruption as a possible dermatological manifestation after Oxford-AstraZeneca vaccine: case report and brief literature review. Dermatol Ther. 2021;34(6):e15129. doi: 10.1111/dth.15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pauluzzi M, Stinco G, Errichetti E. Bullous pemphigoid in a young male after COVID-19 mRNA vaccine: a report and brief literature review. J Eur Acad Dermatol Venereol. 2021;36(4):e257–9. [DOI] [PubMed]
- 8.Yu Phuan CZ, Ci-En Choi E, Oon HH. Temporary exacerbation of pre-existing psoriasis and eczema in the context of COVID-19 mRNA booster vaccination: a case report and review of the literature. JAAD Int. 2021;18(6):94–96. doi: 10.1016/j.jdin.2021.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei N, Kresch M, Elbogen E, Lebwohl M. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022;19:74–77. doi: 10.1016/j.jdcr.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sotiriou E, Tsentemeidou A, Bakirtzi K, Lallas A, Ioannides D, Vakirlis E. Psoriasis exacerbation after COVID-19 vaccination: a report of 14 cases from a single centre. J Eur Acad Dermatol Venereol. 2021;35(12):e857–e859. doi: 10.1111/jdv.17582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perna D, Jones J, Schadt CR. Acute generalized pustular psoriasis exacerbated by the COVID-19 vaccine. JAAD Case Rep. 2021;17:1–3. doi: 10.1016/j.jdcr.2021.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Megna M, Potestio L, Gallo L, Caiazzo G, Ruggiero A, Fabbrocini G. Reply to "Psoriasis exacerbation after COVID-19 vaccination: report of 14 cases from a single centre" by Sotiriou E et al. J Eur Acad Dermatol Venereol. 2022;36(1):E11–E13. doi: 10.1111/jdv.17665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMahon DE, Amerson E, Rosenbach M, Lipoff JB, Moustafa D, Tyagi A, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMahon DE, Kovarik CL, Damsky W, Rosenbach M, Lipoff JB, Tyagi A, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86(1):113–121. doi: 10.1016/j.jaad.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chi C-C, Fang T-P, Lin Y-C. Association of psoriasis with asthma: a systematic review and meta-analysis of observational studies. Dermatol Sin. 2020;38(1):22–27. doi: 10.4103/ds.ds_33_19. [DOI] [Google Scholar]
- 16.Dai YX, Chen TJ, Chang YT. Skin care services and disease prevalence in Taiwan: a nationwide study. Dermatol Sin. 2018;36(3):124–130. doi: 10.1016/j.dsi.2017.11.001. [DOI] [Google Scholar]
- 17.Boehncke W-H, Schön MP. Psoriasis. Lancet. 2015;386(9997):983–994. doi: 10.1016/S0140-6736(14)61909-7. [DOI] [PubMed] [Google Scholar]
- 18.Tsai T-F, Lo Y, Huang Y-H. Long-term effectiveness and safety of ixekizumab in the treatment of 14 patients with a history of chronic erythrodermic psoriasis who failed prior secukinumab: a bicentric retrospective study. Dermatol Sin. 2021;39(2):87. doi: 10.4103/ds.ds_51_20. [DOI] [Google Scholar]
- 19.Rendon A, Schakel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20(6):1475. doi: 10.3390/ijms20061475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wack S, Patton T, Ferris LK. COVID-19 vaccine safety and efficacy in patients with immune-mediated inflammatory disease: review of available evidence. J Am Acad Dermatol. 2021;85(5):1274–1284. doi: 10.1016/j.jaad.2021.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gisondi P, Geat D, Naldi L, Piaserico S. Insights into Sars-CoV-2 vaccination in patients with chronic plaque psoriasis on systemic treatments. J Eur Acad Dermatol Venereol. 2021;35(6):e361–e362. doi: 10.1111/jdv.17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skroza N, Bernardini N, Tolino E, Proietti I, Mambrin A, Marchesiello A, et al. Safety and impact of anti-COVID-19 vaccines in psoriatic patients treated with biologics: a real life experience. J Clin Med. 2021;10(15):3355. doi: 10.3390/jcm10153355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80(10):1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 24.Curtis JR, Johnson SR, Anthony DD, Arasaratnam RJ, Baden LR, Bass AR, et al. American College of Rheumatology Guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 3. Arthritis Rheumatol. 2021;73(10):e60–e75. doi: 10.1002/art.41928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee C-H, Huang Y-H, Chu C-Y. Taiwan dermatological association recommendations for coronavirus disease of 2019 vaccination in patients treated with immunotherapeutics. Dermatol Sin. 2021;39(4):169–174. doi: 10.4103/ds.ds_50_21. [DOI] [Google Scholar]
- 26.Chao J-P, Tsai T-F. Psoriasis flare following ChAdOx1-S/nCoV-19 vaccination in patients with psoriasis under biologic treatment. Dermatol Sin. 2021;39(4):208–209. doi: 10.4103/ds.ds_45_21. [DOI] [Google Scholar]
- 27.Ricardo JW, Lipner SR. Case of de novo nail psoriasis triggered by the second dose of Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;17:18–20. doi: 10.1016/j.jdcr.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pesqué D, Lopez-Trujillo E, Marcantonio O, Giménez-Arnau AM, Pujol RM. New-onset and exacerbations of psoriasis after mRNA COVID-19 vaccines: two sides of the same coin? J Eur Acad Dermatol Venereol. 2022;36(2):e80–e81. doi: 10.1111/jdv.17690. [DOI] [PubMed] [Google Scholar]
- 29.Lehmann M, Schorno P, Hunger RE, Heidemeyer K, Feldmeyer L, Yawalkar N. New onset of mainly guttate psoriasis after COVID-19 vaccination: a case report. J Eur Acad Dermatol Venereol. 2021;35(11):e752–e755. doi: 10.1111/jdv.17561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;29(372):n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gregoire ARF, DeRuyter BK, Stratman EJ. Psoriasis flares following systemic glucocorticoid exposure in patients with a history of psoriasis. JAMA Dermatol. 2021;157(2):198–201. doi: 10.1001/jamadermatol.2020.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song WJ, Lim Y, Jo SJ. De novo guttate psoriasis following coronavirus disease 2019 vaccination. J Dermatol. 2022;49(1):e30–e31. doi: 10.1111/1346-8138.16203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magro C, Crowson AN, Franks L, Schaffer PR, Whelan P, Nuovo G. The histologic and molecular correlates of COVID-19 vaccine-induced changes in the skin. Clin Dermatol. 2021;39(6):966–984. doi: 10.1016/j.clindermatol.2021.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romagnuolo M, Pontini P, Muratori S, Marzano AV, Moltrasio C. De novo annular pustular psoriasis following mRNA COVID-19 vaccine. J Eur Acad Dermatol Venereol. 2022;36(8):e603–5. [DOI] [PubMed]
- 36.Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47(1):153–155. doi: 10.1111/ced.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Catala A, Munoz-Santos C, Galvan-Casas C, Roncero Riesco M, Revilla Nebreda D, Sola-Truyols A, et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186(1):142–152. doi: 10.1111/bjd.20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koumaki D, Krueger-Krasagakis SE, Papadakis M, Katoulis AC, Gkiaouraki I, Zografaki K, et al. Psoriasis flare-up after AZD1222 and BNT162b2 COVID-19 mRNA vaccines: report of twelve cases from a single centre. J Eur Acad Dermatol Venereol. 2022;36(6):e411–5. [DOI] [PubMed]
- 39.Durmaz I, Turkmen D, Altunisik N, Toplu SA. Exacerbations of generalized pustular psoriasis, palmoplantar psoriasis, and psoriasis vulgaris after mRNA COVID-19 vaccine: a report of three cases. Dermatol Ther. 2022;23:e15331. doi: 10.1111/dth.15331. [DOI] [PubMed] [Google Scholar]
- 40.Krajewski PK, Matusiak L, Szepietowski JC. Psoriasis flare-up associated with second dose of Pfizer-BioNTech BNT16B2b2 COVID-19 mRNA vaccine. J Eur Acad Dermatol Venereol. 2021;35(10):e632–e634. doi: 10.1111/jdv.17449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bostan E, Elmas L, Yel B, Yalici-Armagan B. Exacerbation of plaque psoriasis after inactivated and BNT162b2 mRNA COVID-19 vaccines: a report of two cases. Dermatol Ther. 2021;34(6):e15110. doi: 10.1111/dth.15110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yatsuzuka K, Murakami M, Kuroo Y, Fukui M, Yoshida S, Muto J, et al. Flare-up of generalized pustular psoriasis combined with systemic capillary leak syndrome after coronavirus disease 2019 mRNA vaccination. J Dermatol. 2021;49(4):454–8. [DOI] [PubMed]
- 43.Spinelli FR, Favalli EG, Garufi C, Cornalba M, Colafrancesco S, Conti F, et al. Low frequency of disease flare in patients with rheumatic musculoskeletal diseases who received SARS-CoV-2 mRNA vaccine. Arthritis Res Ther. 2022;24(1):21. doi: 10.1186/s13075-021-02674-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quattrini L, Verardi L, Caldarola G, Peluso G, De Simone C, D'Agostino M. New onset of remitting seronegative symmetrical synovitis with pitting oedema and palmoplantar psoriasis flare-up after Sars-Cov-2 vaccination. J Eur Acad Dermatol Venereol. 2021;35(11):e727–e729. doi: 10.1111/jdv.17502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piccolo V, Russo T, Mazzatenta C, Bassi A, Argenziano G, Cutrone M, et al. COVID vaccine-induced pustular psoriasis in patients with previous plaque type psoriasis. J Eur Acad Dermatol Venereol. 2022;36(5):e330–2. [DOI] [PubMed]
- 46.Niebel D, Wenzel J, Wilsmann-Theis D, Ziob J, Wilhelmi J, Braegelmann C. Single-center clinico-pathological case study of 19 patients with cutaneous adverse reactions following covid-19 vaccines. Dermatopathology. 2021;8(4):463–476. doi: 10.3390/dermatopathology8040049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Musumeci ML, Caruso G, Trecarichi AC, Micali G. Safety of SARS-CoV-2 vaccines in psoriatic patients treated with biologics: a real life experience. Dermatol Ther. 2022;35(1):e15177. doi: 10.1111/dth.15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mieczkowska K, Kaubisch A, McLellan BN. Exacerbation of psoriasis following COVID-19 vaccination in a patient previously treated with PD-1 inhibitor. Dermatol Ther. 2021;34(5):e15055. doi: 10.1111/dth.15055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahil SK, Bechman K, Raharja A, Domingo-Vila C, Baudry D, Brown MA, et al. Humoral and cellular immunogenicity to a second dose of COVID-19 vaccine BNT162b2 in people receiving methotrexate or targeted immunosuppression: a longitudinal cohort study. Lancet Rheumatol. 2022;4(1):e42–e52. doi: 10.1016/S2665-9913(21)00333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010. doi: 10.3389/fmed.2021.812010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burlando M, Herzum A, Micalizzi C, Cozzani E, Parodi A. Cutaneous reactions to COVID-19 vaccine at the dermatology primary care. Immun Inflamm Dis. 2022;10(2):265–271. doi: 10.1002/iid3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kabbani M, Poskin M, Benhadou F. Psoriasis exacerbation after COVID-19 vaccination in high-risk group: how to manage it? Dermatol Ther. 2022;9:e15368. doi: 10.1111/dth.15368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nia AM, Silva MM, Spaude J, Gonzalez-Fraga JD. Erythrodermic psoriasis eruption associated with SARS-CoV-2 vaccination. Dermatol Ther. 2022;15:e15380. doi: 10.1111/dth.15380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frioui R, Chamli A, Zaouak A, Hlel I, Khanchel F, Fenniche S, et al. A case of new-onset acute generalized pustular psoriasis following PFIZER-Biontech COVID-19 vaccine. Dermatol Ther. 2022;13:e15444. doi: 10.1111/dth.15444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lopez ED, Javed N, Upadhyay S, Shekhar R, Sheikh AB. Acute exacerbation of psoriasis after COVID-19 Pfizer vaccination. Proc (Bayl Univ Med Cent). 2022;35(2):199–201. doi: 10.1080/08998280.2021.2003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pavia G, Gargiulo L, Spinelli F, Avagliano J, Valenti M, Borroni RG, et al. Generalized pustular psoriasis flare in a patient affected by plaque psoriasis after BNT162b2 mRNA COVID-19 vaccine, successfully treated with risankizumab. J Eur Acad Dermatol Venereol. 2022;17:1–3. [DOI] [PMC free article] [PubMed]
- 57.Rouai M, Slimane MB, Sassi W, Alaoui F, Chelly I, Mokni M. Pustular rash triggered by Pfizer-BioNTech COVID19 vaccination: a case report. Dermatol Ther. 2022;22:e15465. doi: 10.1111/dth.15465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tran TB, Pham NTU, Phan HN, Nguyen HT. Generalized erythrodermic psoriasis triggered by vaccination against severe acute respiratory syndrome coronavirus 2. Dermatol Ther. 2022;20:e15464. doi: 10.1111/dth.15464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fang WC, Chiu LW, Hu SC. Psoriasis exacerbation after first dose of AstraZeneca coronavirus disease 2019 vaccine. J Dermatol. 2021;48(11):e566–e567. doi: 10.1111/1346-8138.16137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Onsun N, Kaya G, Işık BG, Güneş B. A generalized pustular psoriasis flare after CoronaVac COVID-19 vaccination: case report. Health Promot Perspect. 2021;11(2):261–262. doi: 10.34172/hpp.2021.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Durmus O, Akdogan N, Karadag O, Gokoz O. Erythroderma related with the first dose of Pfizer-BioNTech BNT16B2b2 COVID-19 mRNA vaccine in a patient with psoriasis. Dermatol Ther. 2022;8:e15363. doi: 10.1111/dth.15363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Q, Lv C, Han X, Shen M, Kuang Y. A web-based survey on factors for unvaccination and adverse reactions of SARS-CoV-2 vaccines in Chinese patients with psoriasis. J Inflamm Res. 2021;14:6265–6273. doi: 10.2147/JIR.S341429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamazaki F. Psoriasis: comorbidities. J Dermatol. 2021;48(6):732–740. doi: 10.1111/1346-8138.15840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gunes AT, Fetil E, Akarsu S, Ozbagcivan O, Babayeva L. Possible triggering effect of influenza vaccination on psoriasis. J Immunol Res. 2015;2015:258430. doi: 10.1155/2015/258430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takayama K, Satoh T, Hayashi M, Yokozeki H. Psoriatic skin lesions induced by BCG vaccination. Acta Derm Venereol. 2008;88(6):621–622. doi: 10.2340/00015555-0496. [DOI] [PubMed] [Google Scholar]
- 66.Macias VC, Cunha D. Psoriasis triggered by tetanus-diphtheria vaccination. Cutan Ocul Toxicol. 2013;32(2):164–165. doi: 10.3109/15569527.2012.727936. [DOI] [PubMed] [Google Scholar]
- 67.Pattison E, Harrison BJ, Griffiths CE, Silman AJ, Bruce IN. Environmental risk factors for the development of psoriatic arthritis: results from a case-control study. Ann Rheum Dis. 2008;67(5):672–676. doi: 10.1136/ard.2007.073932. [DOI] [PubMed] [Google Scholar]
- 68.Ewer KJ, Barrett JR, Belij-Rammerstorfer S, Sharpe H, Makinson R, Morter R, et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med. 2021;27(2):270–278. doi: 10.1038/s41591-020-01194-5. [DOI] [PubMed] [Google Scholar]
- 69.Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21(4):195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Awada B, Abdullah L, Kurban M, Abbas O. Comment on 'De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine': possible role for Type I interferons. Clin Exp Dermatol. 2022;47(2):443. doi: 10.1111/ced.14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sadeghinia A, Daneshpazhooh M. Immunosuppressive drugs for patients with psoriasis during the COVID-19 pandemic era. A review. Dermatol Ther. 2021;34(1):e14498. doi: 10.1111/dth.14498. [DOI] [PubMed] [Google Scholar]
- 72.Cristaudo A, Graceffa D, Pimpinelli F, Sperati F, Spoletini G, Bonifati C, et al. Immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in psoriasis patients treated with biologic drugs. J Eur Acad Dermatol Venereol. 2021;36(4):e266–8. [DOI] [PubMed]
- 73.Pacifico A, d'Arino A, Pigatto PDM, Malagoli P, Young Dermatologists Italian N. Damiani G. COVID-19 vaccines do not trigger psoriasis flares in patients with psoriasis treated with apremilast. Clin Exp Dermatol. 2021;46(7):1344–1346. doi: 10.1111/ced.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pahalyants V, Murphy WS, Klebanov N, Lu C, Theodosakis N, Klevens RM, et al. Immunosuppressive biologics did not increase the risk of COVID-19 or subsequent mortality: a retrospective matched cohort study from Massachusetts. J Am Acad Dermatol. 2022;86(1):252–255. doi: 10.1016/j.jaad.2021.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Talamonti M, Galluzzo M. Safety of COVID-19 vaccines in patients with psoriasis undergoing therapy with anti-interleukin agents. Expert Opin Biol Ther. 2021;21(11):1535–1537. doi: 10.1080/14712598.2021.1965985. [DOI] [PubMed] [Google Scholar]
- 76.Kalinke U, Barouch DH, Rizzi R, Lagkadinou E, Tureci O, Pather S, et al. Clinical development and approval of COVID-19 vaccines. Expert Rev Vaccines. 2022;21(5):609–619. doi: 10.1080/14760584.2022.2042257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Walter EB, Talaat KR, Sabharwal C, Gurtman A, Lockhart S, Paulsen GC, et al. Evaluation of the BNT162b2 covid-19 vaccine in children 5 to 11 years of age. N Engl J Med. 2022;386(1):35–46. doi: 10.1056/NEJMoa2116298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Creech CB, Anderson E, Berthaud V, Yildirim I, Atz AM, Melendez Baez I, et al. Evaluation of mRNA-1273 Covid-19 vaccine in children 6 to 11 years of age. N Engl J Med. 2022;386(21):2011–2023. doi: 10.1056/NEJMoa2203315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Callaway E. Russia's fast-track coronavirus vaccine draws outrage over safety. Nature. 2020;584(7821):334–335. doi: 10.1038/d41586-020-02386-2. [DOI] [PubMed] [Google Scholar]
- 80.Ozaras R, Berk A, Ucar DH, Duman H, Kaya F, Mutlu H. Covid-19 and exacerbation of psoriasis. Dermatol Ther. 2020;33(4):e13632. doi: 10.1111/dth.13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Diotallevi F, Campanati A, Radi G, Martina E, Rizzetto G, Barbadoro P, et al. Vaccination against SARS-CoV-2 and psoriasis: the three things every dermatologist should know. J Eur Acad Dermatol Venereol. 2021;35(7):e428–e430. doi: 10.1111/jdv.17256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shahidi-Dadras M, Tabary M, Robati RM, Araghi F, Dadkhahfar S. Psoriasis and risk of the COVID-19: is there a role for angiotensin converting enzyme (ACE)? J Dermatolog Treat. 2020;30:1–2. doi: 10.1080/09546634.2020.1782819. [DOI] [PubMed] [Google Scholar]
- 83.Valencia LS, Weng YY, Chandran NS, Ellie CC. Psoriasis flares and rebound phenomenon following exposure and withdrawal of systemic steroids—a systematic review and meta-analysis. J Am Acad Dermatol. 2022;S0190-9622(22)00036-6. [DOI] [PubMed]
- 84.Schwarz CW, Loft N, Andersen V, Juul L, Zachariae C, Skov L. Are systemic corticosteroids causing psoriasis flare-ups? Questionnaire for Danish dermatologists, gastroenterologists and rheumatologists. Dermatology. 2021;237(4):588–594. doi: 10.1159/000510712. [DOI] [PubMed] [Google Scholar]
- 85.Muik A, Lui BG, Wallisch AK, Bacher M, Muhl J, Reinholz J, et al. Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Science. 2022;375(6581):678–80. [DOI] [PMC free article] [PubMed]
- 86.Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barda N, Dagan N, Cohen C, Hernan MA, Lipsitch M, Kohane IS, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398(10316):2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


