Figure 3. Three-Dimensional Protein Modeling of Newly Discovered PADI3 Variants.
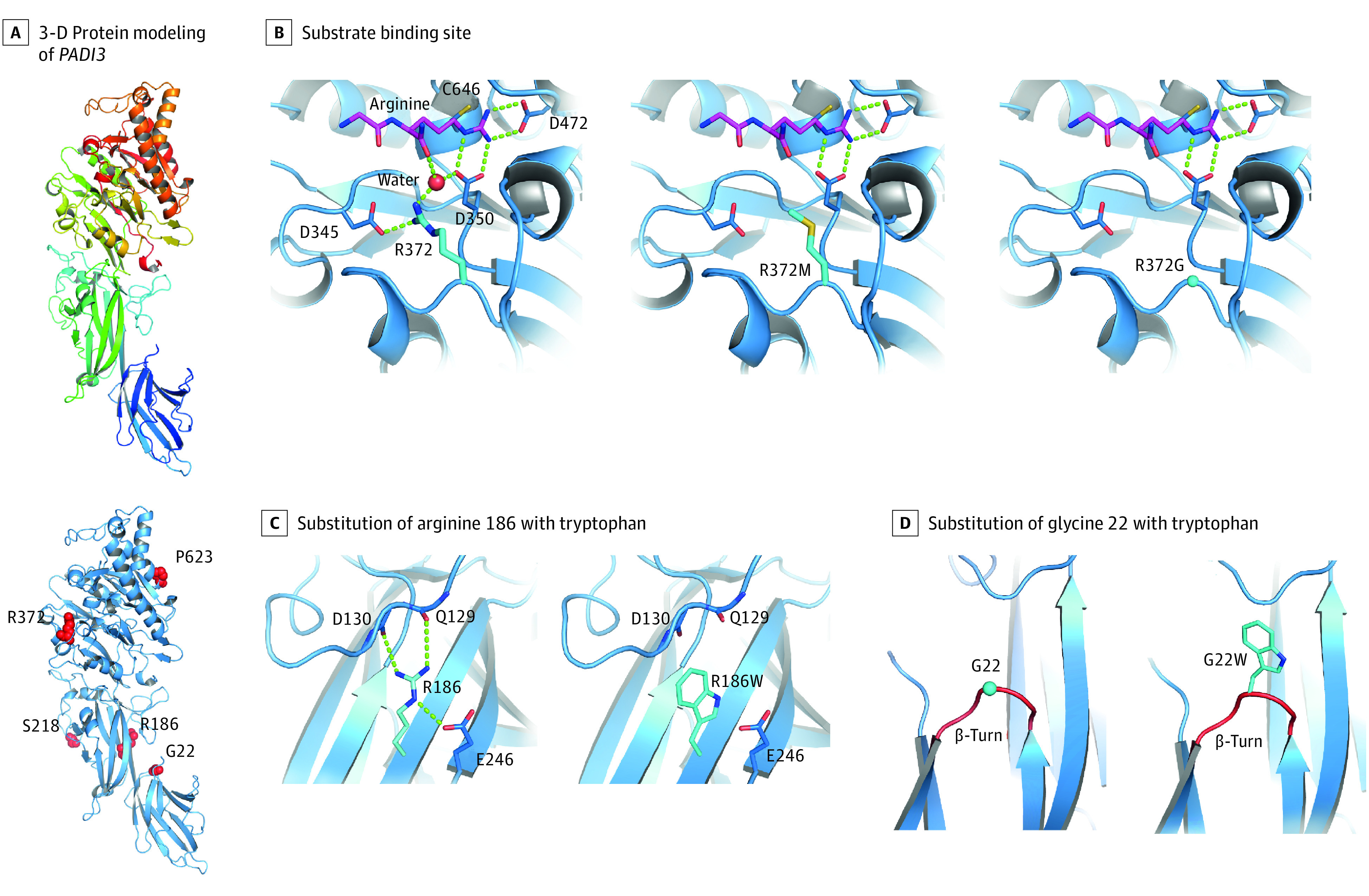
An overview of the entire 3-dimensional model of the wild-type PADI3 gene (A, upper panel) and the locations of the newly identified missense substitutions (A, bottom panel). Arginine 372 is located at the substrate binding site of PADI3 and is involved in the binding of l-arginine residues of target proteins via formation of hydrogen bonds (B, left); its substitution by a methionine (B, middle) or glycine (B, right) is expected to lead to the loss of these hydrogen bonds, thus a weaker substrate binding and lower enzyme activity. Substitution of arginine 186 in the wild-type PADI3 gene (C, left) with a tryptophan (C, right), as well as substitution of glycine 22 (D, left) with a tryptophan (D, right), are expected to destabilize PADI3 owing to loss of stabilizing hydrogen bonds (C) and insertion of a bulky residue in a β-turn loop reducing the flexibility at this site (D).
