Figure 1. Impact of antibiotic exposure in patients with hematologic malignancies treated with anti-CD19 CAR T cell therapy.
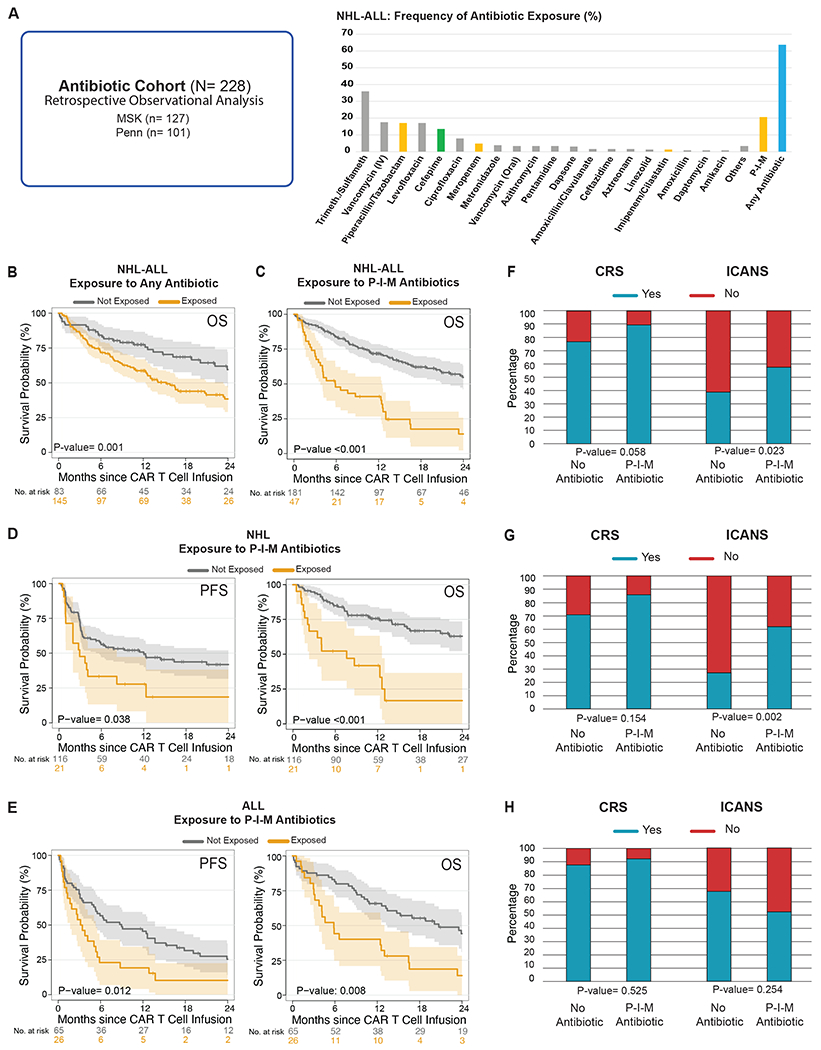
(A) The antibiotic cohort consists of CD19 CAR T cell recipients from MSK (n= 127) and Penn (n= 101) who were assessed in a retrospective observational study of antibiotic exposure (left panel) (N= 228). Frequency of antibiotic exposure in the four weeks prior to CAR T cell infusion in patients with NHL and ALL (right panel). (B) Kaplan-Meier overall survival curves in ALL and NHL populations according to exposure to any antibiotic within the 4 weeks before CD19 CAR T cell infusion (N= 228). (C, D, E) Kaplan-Meier curves of progression-free survival (PFS) and overall survival (OS) according to the exposure to P-I-M antibiotics the within 4 weeks before CD19 CAR T cell infusion in patients with ALL and NHL (only OS, N= 228), NHL (n= 137) and ALL (n= 91), respectively. (B to E) The dark gray line is the estimated Kaplan-Meier survival probability for patients not exposed to P-I-M antibiotics, while the dark yellow line is the estimated probability for patients exposed to P-I-M antibiotics. The shading is the estimated pointwise 95% confidence interval, and the tick marks indicate censored events. (F, G, H) Histograms of the frequencies of any grade CRS and ICANS by Wilcoxon rank-sum test according to exposure to P-I-M antibiotics within 4 weeks before CD19 CAR T cell infusion in patients with ALL and NHL (N= 228), NHL (n= 137) and ALL (n= 91), respectively. Blue indicates the absence of CRS or ICANS of any grade, while red indicates the presence of CRS or ICANS of any grade.
Abbreviations: Trimeth./Sulfameth.: trimethoprim/sulfamethoxazole; IV: intravenous; NHL: non-Hodgkin lymphoma; Not exposed: patients exposed to non-P-I-M plus patients who did not receive any antibiotics within the 4 weeks before CD19 CAR T cell infusion; ALL: acute lymphoblastic leukemia; p: p-value; P-I-M: exposure to either piperacillin/tazobactam, imipenem/cilastatin or meropenem within the 4 weeks before CD19 CAR T cell infusion; CRS: cytokine releasing syndrome; ICANS: immune effector cell-associated neurotoxicity
