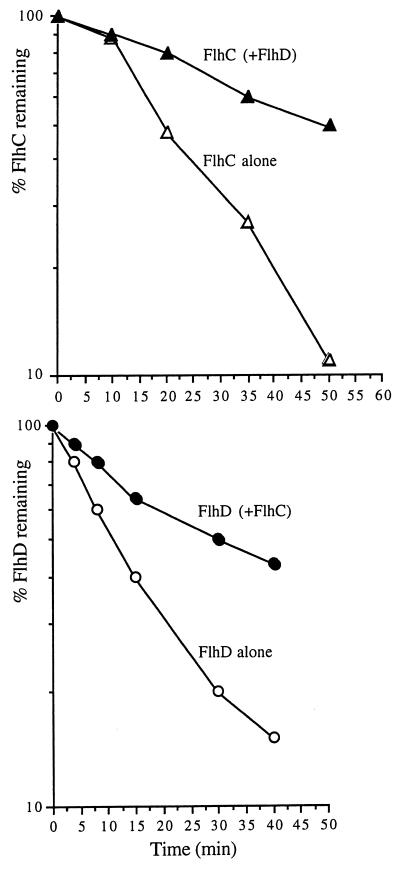
FIG. 3.
Decay of P. mirabilis FlhD and FlhC together or individually from recombinant plasmids pBAD24C, pBAD18D (7), and pBAD18DC (13), which express flhC, flhD, and flhDC, respectively, from an arabinose-inducible promoter in a ΔflhDC::Ω Proteus null mutant (6). Previously unpublished plasmid pBAD24C contains flhC, amplified by PCR using Pfu polymerase, under the control of the arabinose-inducible promoter in the vector pBAD24 (11). Midexponential LB cultures were harvested, washed, and resuspended in minimal medium (M9) containing 0.1% arabinose, 20 μM amino acids (minus methionine), and 1 mM MgSO4. After 5 min of incubation at 37°C, cells were pulse-labeled for 2 min with 20 μCi of [35S]methionine per ml and chased with 2 mM unlabeled methionine and 2% glucose. Equivalent amounts of cells were then removed immediately (time zero) and at intervals, centrifuged, and resuspended in lysis buffer (2% SDS, 50 mM EDTA, 50 mM Tris-HCl [pH 7.4]). After heating at 90°C for 5 min, 20 μl of cell extract was diluted with 1.3 ml of buffer I (50 mM EDTA, 0.1 M NaCl, 0.1% Nonidet P40, 50 mM Tris-HCl [pH 7.4]). The labeled FlhD or FlhC was immunoprecipitated with 5 μl of antiserum (4°C, 90 min). Protein A-Sepharose beads (Sigma) were added (50 μl of a 25% suspension), and after 30 min of incubation at 4°C, precipitates were washed three times with buffer I and once with buffer II (10 mM EDTA, 0.3 M NaCl, 20 mM Tris-HCl [pH 7.4]). Proteins were eluted from beads in loading buffer (5 min, 90°C) and separated by SDS–15% PAGE. Signal strength was detected using a cyclone phosphorimager and quantified using OPTIQUANT software (both from Packard). The experiment was performed three times, and there was no significant variation in the results.