Abstract
Study Objectives:
Supine-predominant obstructive sleep apnea (OSA) is highly prevalent. The proportion of time spent in the supine position may be overrepresented during polysomnography, which would impact on the apnea-hypopnea index (AHI) and have important clinical implications. We aimed to investigate the difference in body position during laboratory or home polysomnography compared to habitual sleep and estimate its effect on OSA severity. Secondary aims were to evaluate the consistency of habitual sleeping position and accuracy of self-reported sleeping position.
Methods:
Patients undergoing diagnostic laboratory or home polysomnography were recruited. Body position was recorded using a neck-worn device. Habitual sleeping position was the average time spent supine over 3 consecutive nights at home. Primary outcomes were the proportion of sleep time spent supine (% time supine) and AHI adjusted for habitual sleeping position.
Results:
Fifty-seven patients who underwent laboratory polysomnography and 56 who had home polysomnography were included. Compared to habitual sleep, % time supine was higher during laboratory polysomnography (mean difference 14.1% [95% confidence interval: 7.2–21.1]; P = .0002) and home polysomnography (7.1% [95% confidence interval 0.9–13.3]; P = .03). Among those with supine-predominant OSA, there was a trend toward lower adjusted AHI than polysomnography-derived AHI (P = .07), changing OSA severity in 31.6%. There was no significant between-night difference in % time supine during habitual sleep (P = .4). Self-reported % time supine was inaccurate (95% limits of agreement –49.2% to 53.9%).
Conclusions:
More time was spent in the supine position during polysomnography compared to habitual sleep, which may overestimate OSA severity for almost one-third of patients with supine-predominant OSA.
Clinical Trial Registration:
Registry: Australia and New Zealand Clinical Trials Registry (ANZCTR); Title: Sleeping position during sleep tests and at home; Identifier: ACTRN12618000628246; URL: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=374873&isReview=true
Citation:
Yo SW, Joosten SA, Wimaleswaran H, et al. Body position during laboratory and home polysomnography compared to habitual sleeping position at home. J Clin Sleep Med. 2022;18(9):2103–2111.
Keywords: sleep apnea syndromes, sleep apnea, obstructive, polysomnography, diagnosis, management
BRIEF SUMMARY
Current Knowledge/Study Rationale: While it is known that the proportion of total sleep time spent in the supine position is an important determinant of sleep apnea severity for the majority of patients, there is a paucity of studies comparing body position during polysomnography and habitual sleep. We investigated the effect of laboratory and home polysomnography on sleeping position and estimated its impact on sleep apnea severity.
Study Impact: This study demonstrated that patients spent a significantly greater proportion of time in the supine position during either modality of polysomnography compared to habitual sleep in the home environment. This may lead to overestimation of sleep apnea severity and has important clinical and research implications for sleep apnea treatment.
INTRODUCTION
Obstructive sleep apnea (OSA) is a highly prevalent disorder associated with deleterious outcomes including excessive daytime sleepiness,1 increased risk of motor vehicle accidents,2 depression,3 hypertension,4 the metabolic syndrome,5 cardiovascular disease,6 and all-cause mortality.7
It is well established that the vast majority of patients with OSA experience more frequent obstructive events in the supine compared to nonsupine sleeping position. Positional OSA, or supine-predominant OSA (spOSA) has been defined using various criteria. The earliest and simplest definition is that proposed by Cartwright8 as a greater than 2-fold difference in the supine vs nonsupine apnea-hypopnea index (AHI), and accounts for approximately 60% of patients with OSA.9–14 A subset of these patients (∼30%) can be defined as having supine-isolated OSA (siOSA),9,11–13 wherein the nonsupine AHI is < 5 events/h, which was first described by Mador and colleagues.13
Patients who undergo laboratory or unattended home polysomnography (PSG) commonly report a discrepancy between body position during PSG and body position during habitual sleep, at least in part due to physical constraints imposed by the PSG equipment. However, patient-perceived body position during PSG is often inaccurate.15–17
Previous small-scale studies suggested that the supine body position is over-represented during laboratory PSG,18,19 and a larger retrospective study demonstrated a similar finding during home PSG.20 These studies employed different methods of recording body position during PSG and habitual sleep, and the study populations were restricted to patients with positional OSA. Apart from these studies, there has been a paucity of data from research employing a systematic and consistent modality of measuring body position to compare PSG and habitual home sleep, and how that relates to patient perception of sleeping position. This is a research and clinical imperative since OSA severity in patients with positional OSA is greatly determined by the proportion of total sleep time spent in the supine position.11,21–23 Therefore, knowledge of the difference in time spent supine between PSG and habitual sleep, and the reliability of patient-perceived sleeping position, would inform clinical interpretation of PSG-recorded AHI and therefore OSA severity. Such nuanced interpretation could enhance treatment recommendation, including but not limited to positional modification devices (PMD), continuous positive airway pressure therapy, and/or mandibular advancement devices.
The objectives of this study were: first, to test the hypothesis that patients spend a greater proportion of sleep time in the supine position during diagnostic laboratory or home PSG compared to habitual sleep; second, to estimate the impact of the difference in body position on OSA diagnostic and severity classification, using a calculated AHI adjusted for habitual body position; and, third, to examine the accuracy of patient perception of body position during habitual sleep.
METHODS
Study population and participants
Adult patients aged 18–85 years undergoing routine diagnostic polysomnography at Monash Medical Centre, Melbourne, Australia, were prospectively screened for inclusion. Two groups of participants were randomly recruited by convenience sampling, based on availability of position-monitoring devices and research staff. Patients who underwent laboratory-attended PSG (in-lab PSG group) were enrolled between May 2018 and August 2018, and those who underwent unattended home PSG (home PSG group) were enrolled from August 2018 through November 2019. Exclusion criteria were: 1) inability to provide informed consent, 2) inability to comprehend instructions for use of the position monitoring device, and 3) mobility-limiting conditions constraining sleeping position.
This project was approved by the Monash Health Human Research and Ethics Committee. All participants gave written informed consent. This trial was prospectively registered at the Australia and New Zealand Clinical Trials Registry (ANZCTR), trial ID ACTRN12618000628246.
Polysomnography
Laboratory-based PSG was performed using Compumedics Grael or E-series equipment (Compumedics, Abbotsford, Victoria, Australia). A standard clinical recording montage was employed. This montage included: electroencephalogram, bilateral electrooculogram, mentalis/submentalis and anterior tibialis electromyogram, electrocardiogram, nasal pressure cannula, oronasal thermistor, thoracic and abdominal respiratory effort bands, and fingertip oximetry. Unattended home level 2 PSG was recorded with Compumedics Somté or Somté PSG, with similar montage except for the absence of oronasal thermistor. For laboratory PSG, body position was measured using a position sensor (Compumedics) placed on the thoracic band approximately midline, which was verified and corrected by continuous video recording. For home PSG, body position was determined by an automatic position sensor (Compumedics) only. The Somté device has an external position sensor and the Somté PSG has an internal position sensor, both with a similar placement on the thoracic band at approximately midline. Patients were asked to sleep in their usual position in the PSG. All PSG were staged and scored manually by experienced sleep scientists using American Academy of Sleep Medicine recommended criteria.24
Home PSG is the most common modality of out-of-center testing at most sleep clinics in Australia and was therefore chosen for the second arm of this study. Limited-channel home sleep apnea testing is infrequently performed in the Australian context.
Position monitoring device
The neck position therapy device (NPTD) (Night Shift; Advanced Brain Monitoring, Carlsbad, CA) is a lightweight device worn over the C7 vertebra spinous process, secured by an adjustable silicone strap. The NPTD acquires real-time data on position and actigraphy-based determination of sleep/wake state, which is recorded in 30-second epochs according to the predominant posture and state in each epoch. Position is reported as supine, lateral left, lateral right, prone, and upright. For position therapy, the device provides vibro-tactile feedback when the supine position is detected. The feedback function was turned off for the purpose of this study, with the NPTD run in “diagnostic” mode only. A detailed description of the NPTD and the validation for supine detection and sleep/wake determination has previously been published.25
Study protocol
Participants wore the NPTD in addition to PSG apparatus on the night of the PSG and were subsequently instructed to wear the device for 3 consecutive nights at home to record habitual sleeping position. Participants were not given specific information on the outcomes of this study and were told to sleep in their usual position.
Participants completed 2 questionnaires, at enrollment into the study and on completion of the study procedures. These consisted of a self-report of the proportion of time spent supine during habitual sleep, on both a 5-point ordinal scale (never to always) and a continuous scale (0%–100%), and the perceived impact of the PSG apparatus on sleep position.
Study outcomes
The primary outcome was the percentage of total sleep time spent in the supine position (% time supine), measured using the NPTD as a continuous variable, during PSG compared to habitual sleep (ie, the NPTD was worn in addition to usual PSG instrumentation during the PSG). The average across the 3 consecutive post-PSG nights at home was used as the measure for habitual sleep. The proportion of participants with an absence of supine sleep (defined as < 30 minutes of NPTD-recorded supine position) during PSG was compared to habitual sleep.
Secondary outcomes included the adjusted AHI (AHIadj) among patients with spOSA and siOSA, which was calculated using the supine AHI, nonsupine AHI and time spent in the supine and nonsupine positions during habitual sleep. The estimation of habitual sleep time was based on actigraphy from the NPTD. The AHIadj was compared to the total AHI (AHItot) determined by PSG. For the purpose of assessing positionality of OSA, participants with < 15 minutes of PSG data in either the supine or nonsupine position were excluded, similar to the approach by previous investigators.13,26 Additional secondary outcomes were the accuracy of participants’ self-reported % time supine during habitual sleep compared to NPTD measurement, and the stability of % time supine across consecutive nights of habitual sleep.
Statistical analysis
Statistical analysis was performed using Stata (Stata 17; StataCorp LLC, College Station, TX). Descriptive statistics were presented as mean ± standard deviation, median (interquartile range [IQR]), or frequency (percentage).
For the primary analysis, the in-lab PSG and home PSG groups were first analyzed separately. PSG compared to habitual % time supine (PSG – habitual supine difference) was evaluated with the paired t test. The proportion with absent supine sleep was compared with McNemar’s test.
A multivariable linear regression model was then fitted to the combined data from both groups to examine effect modification by modality of PSG on the PSG – habitual supine difference, adjusted for age, sex, and mean body mass index. A self-reported difference in sleeping position during PSG (dichotomous variable; spent more time supine than usual vs not) was examined as a predictor of the objectively measured PSG – habitual supine difference, first among all participants, then within each PSG group separately.
Among participants with spOSA, AHIadj was compared to PSG-derived AHItot with the Wilcoxon signed-rank test. The reproducibility of OSA diagnostic and severity classification (no OSA, AHI < 5 events/h; mild OSA, 5 ≤ AHI < 15 events/h; moderate OSA, 15 ≤ AHI < 30 events/h; severe OSA, AHI ≥ 30 events/h)27 between PSG and habitual sleep was evaluated using the kappa (κ) statistic.28
The stability of habitual sleeping position was assessed, first using repeated measures ANOVA to compare % time supine across consecutive nights, and second by examining the analysis of variance of an absence of supine sleep using the κ statistic.
The accuracy of perceived habitual sleeping position was evaluated with a Bland-Altman plot of agreement between self-reported and NPTD-measured % time supine. Further, a 2 × 2 contingency table was constructed and sensitivity, specificity, and predictive values were calculated to compare self-reported habitual sleeping position (never vs any supine sleep) to NPTD recording (using ≥ 30 minutes of measured supine sleep as the threshold for positivity). Last, the predictive utility of this self-report among participants with spOSA was compared to that of participants with nonpositional OSA by means of a multivariable logistic regression model with an interaction term.
A sample size of 56 participants in each of the in-lab PSG and home PSG groups was chosen, allowing for 20% drop-out, which would provide 90% power at the 2-tailed significance level of 5% to detect a within-participant difference of 10% (standard deviation 20%) in the primary outcome of % time supine, comparing PSG to habitual sleep.18,20
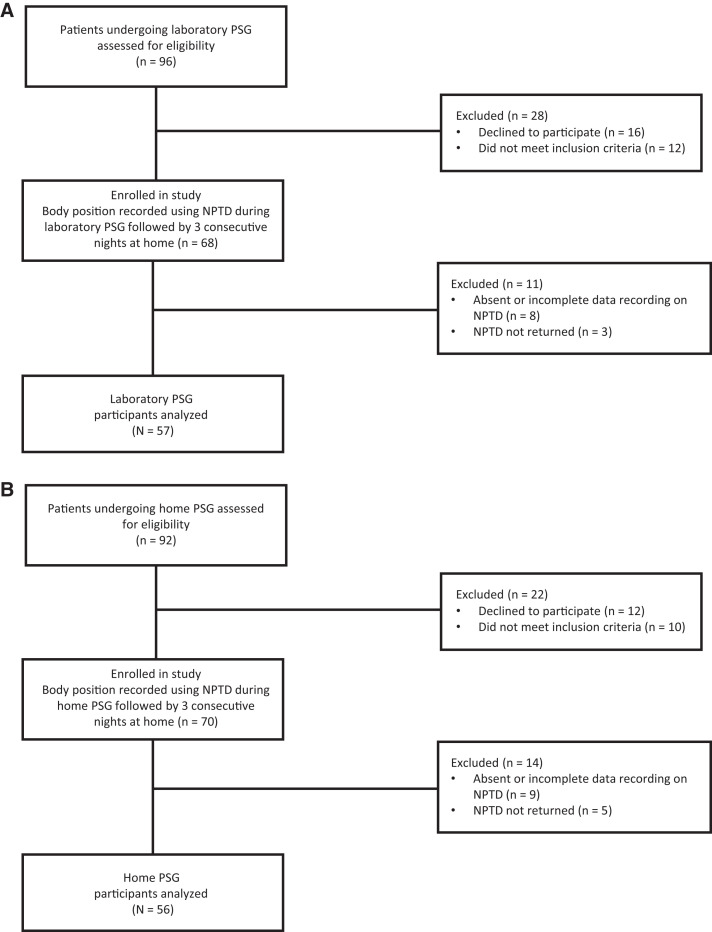
Given minor missing data due to NPTD not being returned or having incomplete data (Figure 1), which were assumed to be missing at random, a complete case method was adopted and participants with missing data were excluded from analysis.
Figure 1. Flow diagram of trial.
(A) Laboratory polysomnogram participants. (B) Home polysomnogram participants. NPTD = neck position therapy device, PSG = polysomnogram.
A P-value of < .05 for 2-sided tests was considered to indicate statistical significance.
RESULTS
Patients
Data were available for 57 in-lab PSG participants and 56 home PSG participants (Figure 1). Baseline characteristics are presented in Table 1. Across both groups (n = 113), mean age was 48.5 ± 13.6 years, 74 (65.5%) were male, 81 (71.7%) were white, and mean body mass index was 32.3 ± 8.5 kg/m2. Notable differences between the groups were a significantly shorter total sleep time among the in-lab PSG participants, 349.0 vs 386.9 minutes (P = .009) and a trend toward a greater proportion of time spent in the supine position during in-lab PSG, 46% vs 37.4% (P = .1). Although the prevalence of OSA was similar between the groups, a larger proportion of the in-lab PSG participants had a diagnosis of severe OSA, 36.8% vs 21.4% (P = .04). Overall, among all participants with OSA, 60% had spOSA and 14% had siOSA, without a significant difference between the in-lab PSG and home PSG groups (P = .4). However, supine AHI were higher in the in-lab PSG group compared to the home PSG group, median 50.5 vs 25.0 events/h (P = .02).
Table 1.
Baseline demographic and clinical characteristics of participants.
| All (n = 113) | In-Lab PSG (n = 57) | Home PSG (n = 56) | P # | |
|---|---|---|---|---|
| Age, years—mean ± SD | 48.5 ± 13.6 | 49.0 ± 13.7 | 47.9 ± 13.7 | .7 |
| Sex, male—n (%) | 74 (65.5) | 37 (64.9) | 37 (66.1) | .9 |
| Race—n (%) | ||||
| White | 81 (71.7) | 43 (75.4) | 38 (67.9) | .5 |
| Asian | 29 (25.7) | 12 (21.1) | 17 (30.4) | |
| Other or missing | 3 (2.7) | 2 (3.5) | 1 (1.8) | |
| Body mass index, kg/m2—mean ± SD | 32.3 ± 8.5 | 32.4 ± 8.9 | 32.2 ± 8.0 | .9 |
| TST, minutes—mean ± SD | 367.8 ± 78.4 | 349.0 ± 9.3 | 386.9 ± 11.0 | .009 |
| Supine sleep, % of TST—mean ± SD | 41.7 ± 29.4 | 46.0 ± 29.9 | 37.4 ± 28.5 | .1 |
| Sleep efficiency, %—mean ± SD | 73.7 ± 15.0 | 75.5 ± 13.9 | 71.8 ± 16.0 | .2 |
| OSA—n (%) | 90 (79.7) | 46 (80.7) | 44 (78.6) | .04 |
| Mild OSA | 29 (25.7) | 17 (29.8) | 12 (21.4) | |
| Moderate OSA | 28 (24.8) | 8 (14.0) | 20 (35.7) | |
| Severe OSA | 33 (29.2) | 21 (36.8) | 12 (21.4) | |
| Minimum oxygen saturation, %—median (IQR) | 85 (78–88) | 86 (79–90) | 83 (77–87) | .5 |
| AHI, events/h—median (IQR) | 17.3 (7.3–34.1) | 15.5 (7.3–49) | 18.8 (7.2–27.6) | .1 |
| AHIS, events/h—median (IQR) | 29.1 (8.3–72.4) | 50.5 (7.5–77.8) | 25.0 (8.6–45.0) | .02 |
| n = 83* | n = 43* | n = 40* | ||
| AHINS, events/h—median (IQR) | 9.7 (1.5–28.1) | 11.4 (1.4–30.3) | 9.5 (2.0–20.5) | .5 |
| n = 83* | n = 43* | n = 40* | ||
| AHIS/AHINS ratio—median (IQR) | 2.1 (1.3–4.9) | 2.5 (1.4–5.4) | 1.9 (0.9–2.9) | .08 |
| n = 83* | n = 43* | n = 40* | ||
| spOSA proportion of OSA (%) | 38/63 (60.3) | 21/32 (65.6) | 17/31 (54.8) | .4 |
| n = 83* | n = 43* | n = 40* | ||
| siOSA proportion of OSA (%) | 9/63 (14.3) | 6/32 (18.8) | 3/31 (9.7) | .3 |
| n = 83* | n = 43* | n = 40* |
*Participants with ≥ 15 minutes of both supine and nonsupine sleep during PSG. #P-values for comparison between in-lab PSG and at-home PSG groups. AHI = apnea-hypopnea index, AHINS = nonsupine AHI, AHIN = supine AHI, IQR = interquartile range, OSA = obstructive sleep apnea, PSG = polysomnography, SD = standard deviation, siOSA = supine-isolated OSA, spOSA = supine-predominant OSA, TST = total sleep time.
Body position during polysomnography compared to habitual sleep
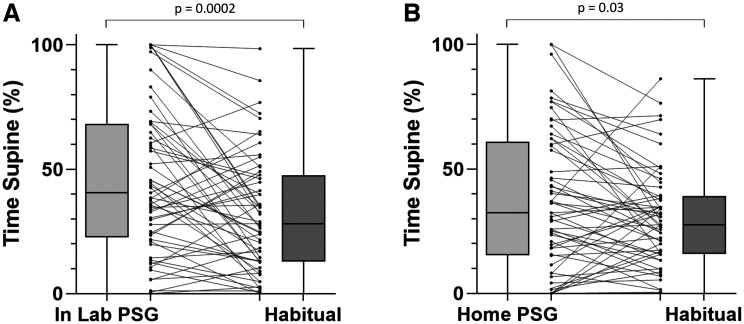
Among the in-lab PSG group, NPTD-recorded % time supine decreased from 46.0 ± 29.9% during PSG to 31.8 ± 23.6% during habitual sleep, a mean difference of 14.1% (95% confidence interval 7.2%–21.1%, P = .0002) (Figure 2A). Seven participants (12.3%) had an absence of supine sleep during in-lab PSG, compared to 9 (15.8%) during habitual sleep (P = .7).
Figure 2. Percent of total sleep time in the supine position, polysomnogram vs habitual sleep.
(A) Laboratory polysomnogram participants. (B) Home polysomnogram participants. PSG = polysomnography.
Participants in the home PSG group spent 37.4 ± 28.5% in the supine position during PSG compared to 30.3 ± 19.9% during habitual sleep, mean difference 7.1% (95% confidence interval 0.9%–13.3%, P = .03) (Figure 2B). An absence of supine sleep was observed in 11 participants (19.6%) during home PSG, compared to 7 (12.5%) during habitual sleep (P = .3).
The PSG – habitual % time supine difference was greater in the in-lab PSG group compared to the home PSG group, 14.1% vs 7.1%, although this did not reach statistical significance, both in the unadjusted and adjusted models (P = .1 for both).
Participants who reported perceiving more time spent in the supine position than usual due to the PSG apparatus had a significantly larger PSG – habitual % time supine difference, 19.9% compared to a difference of 6.1% among those who did not (P = .009). This question only had predictive utility in the in-lab PSG group, wherein participants who responded “yes” had a PSG – habitual % time supine difference of 31.1%, compared to 4% for the home PSG group (P = .01 for interaction).
Consistency of habitual sleeping position and accuracy of self-report
Overall, participants spent a median of 28.1% (IQR 13.4%–44.6%) in the supine position during habitual sleep, averaged across 3 consecutive nights. This was stable night-to-night (P = .4 for between-night difference). There was substantial agreement between nights for the absence of supine sleep, which ranged from 21%–27% of participants across the nights, κ statistic 0.72 (P < .0001).
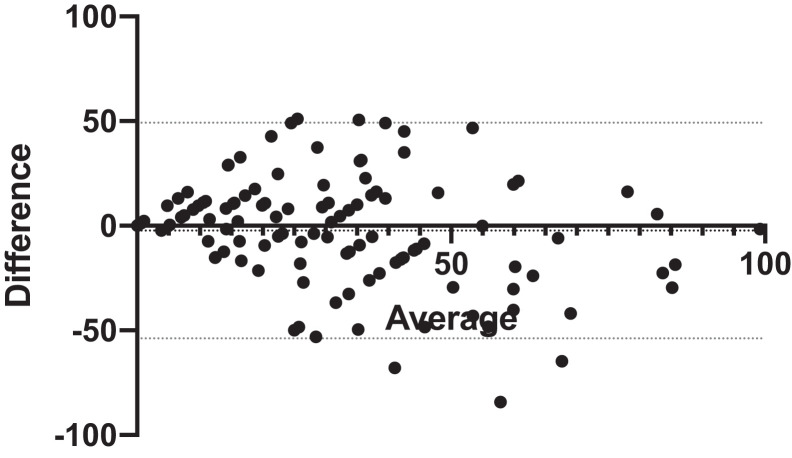
Bland-Altman analysis showed a mean bias of 2.3% between self-reported and measured habitual % time supine, with 95% limits of agreement of –49.2% to 53.9 (Figure 3). Of the 18 participants who did not perceive any habitual supine sleep, 6 had no measured supine sleep, negative predictive value 33.3%. Conversely, 85 of the 95 participants who perceived any habitual supine sleep had supine sleep recorded with the NPTD, positive predictive value 89.5%. Accordingly, sensitivity and specificity of the self-report were 87.6% and 37.5%, respectively. Among participants with spOSA (n = 38), the negative predictive value of a perceived absence of habitual supine sleep was 75%, compared to 25% for those with nonpositional OSA (P = .05 for interaction).
Figure 3. Bland-Altman plot: self-reported vs NPTD-measured percent of total sleep time in the supine position during habitual sleep in the home environment.
Dotted lines: 95% limits of agreement. NPTD = neck position therapy device.
Reliability of OSA diagnostic classification in patients with positional OSA
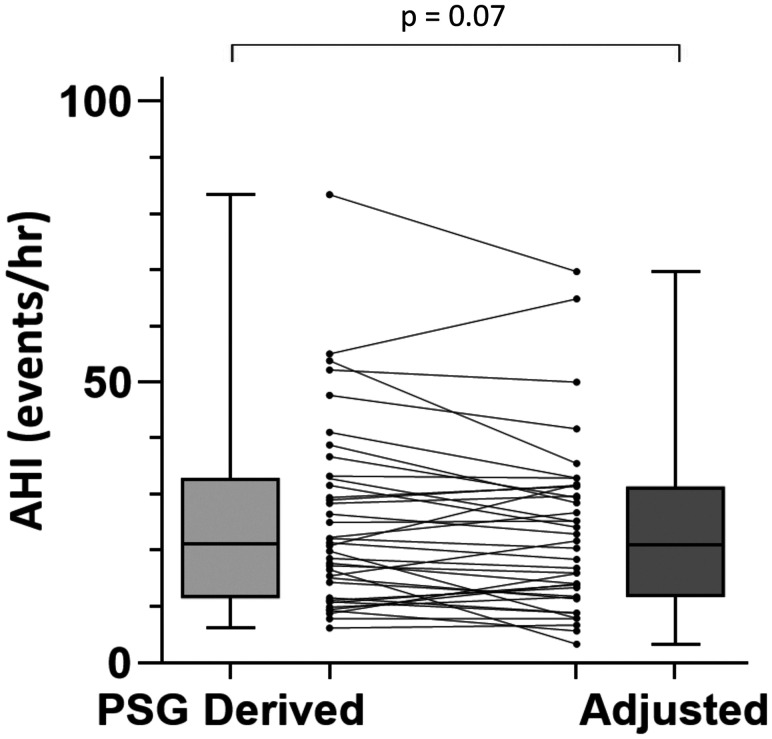
Among the 38 participants with spOSA, median AHItot was 21.1 events/h (IQR 11.6–32.8 events/h) and median AHIadj was 21.1 events/h (IQR 11.8–31.4 events/h), with a trend toward a lower AHIadj, P = .07 (Figure 4). There was only moderate reproducibility of PSG-determined OSA diagnosis category after accounting for habitual sleeping position, raw agreement 68.4%, κ 0.53 (P < .0001).
Figure 4. PSG-derived vs adjusted AHI, among patients with supine-predominant OSA.
AHI = apnea-hypopnea index, OSA = obstructive sleep apnea, PSG = polysomnography.
DISCUSSION
To our knowledge, this is the first study to systematically examine the effect of both laboratory and home PSG on inducing a change in body position during sleep, using a consistent and validated method to record position across the different sleeping conditions.
The results from the present study provide evidence that patients spend a significantly greater proportion of sleep time in the supine position during PSG compared to habitual sleep. Laboratory PSG appeared to have a larger effect than home PSG on inducing a change in body position compared to habitual, although this did not reach statistical significance.
In patients undergoing a laboratory PSG, the perception of more time spent supine than usual predicted a greater proportion of time spent supine during the PSG than habitual sleep. This was not seen for those who underwent home PSG. Finally, within the subgroup of patients with spOSA, PSG-derived total AHI may overestimate OSA severity classification for almost one-third of patients, due to an over-representation of supine sleep during PSG compared to usual. The trend toward a lower AHIadj and the relatively high rate of potential OSA severity misclassification, in spite of a similar median (IQR) AHItot and AHIadj at a group-level, is explained by the paired nature of the data, and a moderate degree of within-participant difference in AHItot and AHIadj (Figure 4).
Our results are consistent with and extend previous studies that individually compared laboratory PSG to habitual sleeping position and home PSG to habitual sleeping position. In a small randomized controlled crossover trial of 15 patients using a chest-worn PMD, Bignold and colleagues demonstrated a significantly greater time spent supine during laboratory PSG compared to home sleep during the “inactive” treatment condition, when the PMD vibration feedback was switched off, 36.6% vs 19.3%.18
Vonk and colleagues conducted a retrospective study of 168 patients with spOSA who had undergone home PSG and were prescribed a chest-worn PMD. During the first 2 nights of treatment initiation vibration feedback was inactive. Patients spent 43.1% of total recording time in the supine position during home PSG, compared to 28.6% during the inactive phase of PMD treatment initiation.20 They reported that 39% of patients had a change in OSA severity classification on the basis of AHI adjusted for habitual sleeping position,20 a finding that was near identical to the effect measured in our study. This is not unexpected given that the amount of time spent supine has been demonstrated to be a major determinant of OSA severity, particularly among patients with positional OSA.21–23
We present the novel finding that habitual body position during sleep has substantial night-to-night repeatability, and this includes the consistent finding of an absence of supine sleep in over one-fifth of patients. However, our results demonstrate that patient-perceived habitual sleeping position has poor accuracy, and self-report of an absence of supine sleep is often unreliable. Patients with spOSA appear to be more accurate at perceiving a lack of supine sleep than those with nonpositional OSA; however, this was an exploratory outcome based on a small subgroup of patients and should be regarded with caution. Previous studies have demonstrated the inaccuracy of patient-perceived sleeping position during laboratory PSG15,16 and home testing,17 and our results extend these findings to habitual sleep in the home environment.
In light of these results, the effect of PSG on inducing an increase in supine sleep and therefore potentially overestimating OSA severity should be considered by clinicians when managing patients with positional OSA. While continuous positive airway pressure remains the standard of care for patients with severe OSA, positional therapy offers an attractive treatment modality for patients with spOSA, particularly since the advent of electronic position modification devices which use vibro-tactile feedback to reduce both supine sleep and in turn OSA severity.29,30 Such devices tend to have better patient acceptability and tolerability compared to continuous positive airway pressure, and thus potentially superior long-term treatment adherence.31
For patients with spOSA who self-report sleeping only in the nonsupine position habitually, a suggested approach would be to document this objectively with a position monitoring device given the inaccuracy of patient perception. Our data show that over one-fifth of patients habitually do not sleep in the supine position, and this is consistent night-to-night. Therefore, objective confirmation of the absence of supine sleep in the home environment may indicate that no specific intervention is required for this subgroup of patients with siOSA, other than regular follow up for monitoring of symptoms and consideration for repeat testing, in light of recent evidence that some patients with siOSA progress to having nonpositional OSA over time.32,33
A number of limitations of this study should be considered in the interpretation of these results. First, head position can influence OSA severity34,35; however, this was not measured in our study. Second, in the calculation of the adjusted AHI based on habitual sleeping position, the NPTD actigraphy-determined sleep vs wake state was used, and the influence of variability in NREM vs REM sleep proportion could not be accounted for. Although sleep stage has been shown to be a less important determinant of overall AHI than body position,11,23 this impacts on the validity of the adjusted AHI outcome, and the comparison between adjusted AHI and PSG-derived AHI should be deemed exploratory. Third, our finding of consistency of habitual sleeping position was based on a short period of observation of 3 consecutive nights. Longer-term studies are required to determine if repeatability remains over longer durations, or if this was merely a short-term effect. Finally, our results from the home PSG group would not be generalizable to populations in jurisdictions where limited-channel home sleep apnea testing (HSAT) is performed in lieu of home PSG. HSAT equipment is often less obtrusive than level 2 PSG and may have a smaller impact on body position; however, this hypothesis would need to be tested in a future study. Nonetheless, the results of the primary outcome in the laboratory PSG group of the present study have broad clinical relevance.
The strengths of this study are, first, the objective measure of body position using the same method of measurement during PSG and habitual sleep. Second, the small, neck-worn position monitoring apparatus is less likely to affect habitual sleeping position compared to bulkier chest-worn devices used by previous investigators. Third, the results from our study population are generalizable to the broader population of patients undergoing PSG at large tertiary sleep centers in Australia, although caution should be taken if extrapolating our findings to other patient populations with different demographics or a higher prevalence of severe obesity. Fourth, although participants were aware that the NPTD was a body position sensor, they were not made cognizant of the primary study outcome. Participants were instructed to sleep in their usual body position during the PSG, consistent with the routine clinical approach at our sleep laboratory, and similarly for the subsequent 3 nights of habitual sleep with the NPTD. In contrast, previous studies had included only patients with positional OSA, who had knowledge of their diagnosis, which may have induced them to avoid the supine position, thus biasing the comparison of PSG to home sleeping position. Last, multiple nights of habitual sleep were sampled to obtain a representative estimate of habitual sleeping position.
In conclusion, the results of this study demonstrate that PSG influences sleeping position. This effect may be larger for laboratory PSG than home PSG. This finding has important clinical and research implications for positional OSA, a phenotype which comprises two-thirds of the OSA patient population. Habitual sleeping position, and importantly the absence of supine sleep, is consistent across nights in the majority of patients; however, patient perception of habitual sleeping position is often inaccurate. Therefore, self-reports of an absence of supine sleep should be verified by objective assessment to inform major clinical treatment decisions for patients with positional OSA.
ACKNOWLEDGMENTS
The authors thank the participants of the study, the sleep laboratory staff at Monash Medical Centre, and the individuals who assisted with recruitment—Hugh Buzacott, Sheetal Deshpande, Timothy Cheung, Claire Serraglio, Cheryl Lim, and Grace Yap.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The study was performed at Monash Medical Centre, Clayton, VIC, Australia. Funding for purchasing the neck positional therapy devices was awarded by the Monash Lung and Sleep Institute (MLSI). B.A.E. received research support from Apnimed Australia and personal fees from Signifier Medical outside the scope of the current work. G.S.H. received equipment to support research from Philips Respironics, ResMed, and Air Liquide Healthcare. The other authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AHIadj
adjusted apnea-hypopnea index
- AHItot
total apnea-hypopnea index
- IQR
interquartile range
- NPTD
neck position therapy device
- OSA
obstructive sleep apnea
- PMD
positional modification devices
- PSG
polysomnography
- siOSA
supine-isolated obstructive sleep apnea
- spOSA
supine-predominant obstructive sleep apnea
REFERENCES
- 1. Gottlieb DJ , Whitney CW , Bonekat WH , et al . Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study . Am J Respir Crit Care Med. 1999. ; 159 ( 2 ): 502 – 507 . [DOI] [PubMed] [Google Scholar]
- 2. Tregear S , Reston J , Schoelles K , Phillips B . Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis . J Clin Sleep Med. 2009. ; 5 ( 6 ): 573 – 581 . [PMC free article] [PubMed] [Google Scholar]
- 3. Peppard PE , Szklo-Coxe M , Hla KM , Young T . Longitudinal association of sleep-related breathing disorder and depression . Arch Intern Med. 2006. ; 166 ( 16 ): 1709 – 1715 . [DOI] [PubMed] [Google Scholar]
- 4. Peppard PE , Young T , Palta M , Skatrud J . Prospective study of the association between sleep-disordered breathing and hypertension . N Engl J Med. 2000. ; 342 ( 19 ): 1378 – 1384 . [DOI] [PubMed] [Google Scholar]
- 5. Coughlin SR , Mawdsley L , Mugarza JA , Calverley PM , Wilding JP . Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome . Eur Heart J. 2004. ; 25 ( 9 ): 735 – 741 . [DOI] [PubMed] [Google Scholar]
- 6. Shamsuzzaman ASM , Gersh BJ , Somers VK . Obstructive sleep apnea: implications for cardiac and vascular disease . JAMA. 2003. ; 290 ( 14 ): 1906 – 1914 . [DOI] [PubMed] [Google Scholar]
- 7. Marshall NS , Wong KKH , Liu PY , Cullen SRJ , Knuiman MW , Grunstein RR . Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study . Sleep. 2008. ; 31 ( 8 ): 1079 – 1085 . [PMC free article] [PubMed] [Google Scholar]
- 8. Cartwright RD . Effect of sleep position on sleep apnea severity . Sleep. 1984. ; 7 ( 2 ): 110 – 114 . [DOI] [PubMed] [Google Scholar]
- 9. Joosten SA , Hamza K , Sands S , Turton A , Berger P , Hamilton G . Phenotypes of patients with mild to moderate obstructive sleep apnoea as confirmed by cluster analysis . Respirology. 2012. ; 17 ( 1 ): 99 – 107 . [DOI] [PubMed] [Google Scholar]
- 10. Joosten SA , O’Donoghue FJ , Rochford PD , et al . Night-to-night repeatability of supine-related obstructive sleep apnea . Ann Am Thorac Soc. 2014. ; 11 ( 5 ): 761 – 769 . [DOI] [PubMed] [Google Scholar]
- 11. Gillman A , Roebuck T , Ho S , van Braak E , Naughton MT . Comparison of supine-only and REM-only obstructive sleep apnoea . Sleep Med. 2012. ; 13 ( 7 ): 875 – 878 . [DOI] [PubMed] [Google Scholar]
- 12. Heinzer R , Petitpierre NJ , Marti-Soler H , Haba-Rubio J . Prevalence and characteristics of positional sleep apnea in the HypnoLaus population-based cohort . Sleep Med. 2018. ; 48 : 157 – 162 . [DOI] [PubMed] [Google Scholar]
- 13. Mador MJ , Kufel TJ , Magalang UJ , Rajesh SK , Watwe V , Grant BJB . Prevalence of positional sleep apnea in patients undergoing polysomnography . Chest. 2005. ; 128 ( 4 ): 2130 – 2137 . [DOI] [PubMed] [Google Scholar]
- 14. Ravesloot MJL , Frank MH , van Maanen JP , Verhagen EA , de Lange J , de Vries N . Positional OSA part 2: retrospective cohort analysis with a new classification system (APOC) . Sleep Breath. 2016. ; 20 ( 2 ): 881 – 888 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wallbridge PD , Churchward TJ , Worsnop CJ . Accuracy of patient perception of supine sleep . J Clin Sleep Med. 2018. ; 14 ( 7 ): 1205 – 1208 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Russo K , Bianchi MT . How reliable is self-reported body position during sleep? J Clin Sleep Med. 2016. ; 12 ( 1 ): 127 – 128 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sorscher AJ , Anzivino AP , Mackenzie T . Patient-predicted sleep position vs. HST data: a tendency to underestimate supine sleep . Sleep Breath. 2018. ; 22 ( 3 ): 625 – 630 . [DOI] [PubMed] [Google Scholar]
- 18. Bignold JJ , Mercer JD , Antic NA , McEvoy RD , Catcheside PG . Accurate position monitoring and improved supine-dependent obstructive sleep apnea with a new position recording and supine avoidance device . J Clin Sleep Med. 2011. ; 7 ( 4 ): 376 – 383 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Metersky ML , Castriotta RJ . The effect of polysomnography on sleep position: possible implications on the diagnosis of positional obstructive sleep apnea . Respiration. 1996. ; 63 ( 5 ): 283 – 287 . [DOI] [PubMed] [Google Scholar]
- 20. Vonk PE , de Vries N , Ravesloot MJL . Polysomnography and sleep position, a Heisenberg phenomenon?: A large-scale series . HNO. 2019. ; 67 ( 9 ): 679 – 684 . [DOI] [PubMed] [Google Scholar]
- 21. Sunnergren O , Broström A , Svanborg E . Positional sensitivity as a confounder in diagnosis of severity of obstructive sleep apnea . Sleep Breath. 2013. ; 17 ( 1 ): 173 – 179 . [DOI] [PubMed] [Google Scholar]
- 22. Yalciner G , Babademez MA , Gul F . Association of sleep time in supine position with apnea-hypopnea index as evidenced by successive polysomnography . Sleep Breath. 2017. ; 21 ( 2 ): 289 – 294 . [DOI] [PubMed] [Google Scholar]
- 23. Eiseman NA , Westover MB , Ellenbogen JM , Bianchi MT . The impact of body posture and sleep stages on sleep apnea severity in adults . J Clin Sleep Med. 2012. ; 8 ( 6 ): 655 – 66A . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berry RB , Brooks R , Gamaldo CE , et al. ; for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.2. Darien, IL: : American Academy of Sleep Medicine; ; 2015. . [Google Scholar]
- 25. Levendowski DJ , Seagraves S , Popovic D , Westbrook PR . Assessment of a neck-based treatment and monitoring device for positional obstructive sleep apnea . J Clin Sleep Med. 2014. ; 10 ( 8 ): 863 – 871 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Joosten SA , O’Driscoll DM , Berger PJ , Hamilton GS . Supine position related obstructive sleep apnea in adults: pathogenesis and treatment . Sleep Med Rev. 2014. ; 18 ( 1 ): 7 – 17 . [DOI] [PubMed] [Google Scholar]
- 27. Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine . Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults . J Clin Sleep Med. 2009. ; 05 ( 03 ): 263 – 276 . [PMC free article] [PubMed] [Google Scholar]
- 28. Landis JR , Koch GG . The measurement of observer agreement for categorical data . Biometrics. 1977. ; 33 ( 1 ): 159 – 174 . [PubMed] [Google Scholar]
- 29. Barnes H , Edwards BA , Joosten SA , Naughton MT , Hamilton GS , Dabscheck E . Positional modification techniques for supine obstructive sleep apnea: A systematic review and meta-analysis . Sleep Med Rev. 2017. ; 36 : 107 – 115 . [DOI] [PubMed] [Google Scholar]
- 30. Ravesloot MJL , White D , Heinzer R , Oksenberg A , Pépin JL . Efficacy of the new generation of devices for positional therapy for patients with positional obstructive sleep apnea: A systematic review of the literature and meta-Analysis . J Clin Sleep Med. 2017. ; 13 ( 6 ): 813 – 824 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Maanen JP , de Vries N . Long-term effectiveness and compliance of positional therapy with the sleep position trainer in the treatment of positional obstructive sleep apnea syndrome . Sleep. 2014. ; 37 ( 7 ): 1209 – 1215 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oksenberg A , Goizman V , Eitan E , Nasser K , Gadoth N , Leppänen T . Obstructive sleep apnea: Do positional patients become nonpositional patients with time? Laryngoscope. 2020. ; 130 ( 9 ): 2263 – 2268 . [DOI] [PubMed] [Google Scholar]
- 33. Chang WH , Wu HC , Lan CC , Wu YK , Yang MC . The worsening of positional mild obstructive sleep apnea over time is associated with an increase in body weight: impact on blood pressure and autonomic nervous system . Respiration. 2021. ; 100 ( 11 ): 1060 – 1069 . [DOI] [PubMed] [Google Scholar]
- 34. Zhu K , Bradley TD , Patel M , Alshaer H . Influence of head position on obstructive sleep apnea severity . Sleep Breath. 2017. ; 21 ( 4 ): 821 – 828 . [DOI] [PubMed] [Google Scholar]
- 35. van Kesteren ER , van Maanen JP , Hilgevoord AAJ , Laman DM , de Vries N . Quantitative effects of trunk and head position on the apnea hypopnea index in obstructive sleep apnea . Sleep. 2011. ; 34 ( 8 ): 1075 – 1081 . [DOI] [PMC free article] [PubMed] [Google Scholar]