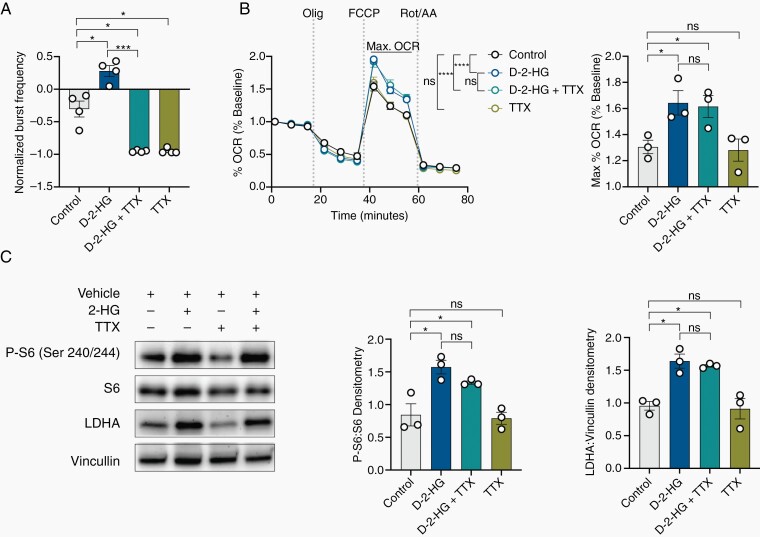
Fig. 5.
d-2-HG induces mTOR upregulation and metabolic reprogramming independent of neuronal bursting. (A) Tetrodotoxin (TTX) silences all neuronal firing compared to control (n = 4, t(3) = 5.792, P = .0102, paired t test) even with co-treatment of d-2-HG (n = 4; control vs d-2-HG + TTX, t(3) = 5.312, P = .0130, paired t test; d-2-HG vs d-2-HG +TTX, t(3) = 16.06, P = .0005, paired t test). d-2-HG without TTX increases normalized burst frequency compared to control (n = 4, t(3) = 4.164, P = .0252, paired t test). (B) d-2-HG increases maximal respiration compared to control and in the setting of TTX (n = 4, F(33, 356) = 5.319, P < .0001, ns: not significant, Two-way ANOVA with Sidak’s multiple comparisons test). (C) Averaged across time points exhibiting maximal respiration, d-2-HG increases max OCR compared to control (n = 3, t(2) = 5.719, P = .0292, paired t test) even with neuronal silencing in the presence of TTX (n = 3, t(2) = 0.2604, P = .8189, paired t test). TTX did not change maximal respiration compared to control (n = 3, t(2) = 0.5140, P = .6584, paired t test). C) Western blot analysis demonstrates that d-2-HG continues to upregulate P-S6:S6 (n = 3, control vs d-2-HG + TTX, t(2) = 3.413, P = .0454, paired t test) and LDHA:Vinculin (n = 3, mean ±SEM, control vs d-2-HG + TTX, t(2) = 12.56, P = .0063, paired t test) in the setting of TTX.