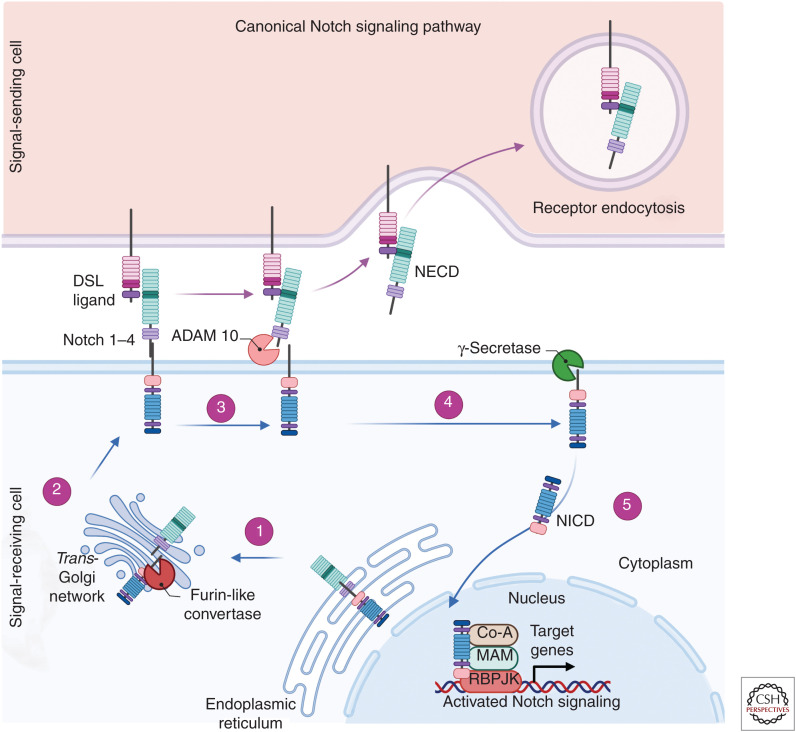
Figure 1.
Schematic of the canonical Notch signaling pathway. (1) Notch receptors (1–4) are initially synthesized as single-chain precursors within the endoplasmic reticulum and subsequently transported to the Golgi apparatus. (2) The Notch precursor then undergoes cleavage by furin forming an extracellular and transmembrane domain and is modified by the glycotransferases fringe. These modified proteins are transported and inserted into the cell membrane. The Notch receptor interacts with Delta/Serrate/Lag (DSL)-2 ligands Jagged 1, Jagged 2, Delta-like 1 (Dll1), Delta-like 3 (Dll3), or Delta-like 4 (Dll4). (3) Upon binding, the Notch receptor is cleaved by ADAM 10 releasing the Notch extracellular domain (NECD) that is then degraded by receptor endocytosis. (4) There is then a subsequent proteolytic cleavage by γ-secretase releasing the Notch intracellular domain (NICD) into the cytoplasm. (5) The NICD is then translocated to the nucleus and forms a coactivator complex with factors including recombination signal-binding protein for immunoglobulin κJ (RBPJK) and Mastermind-inducing transcription of Hes and Hey genes. (Created in BioRender.com.)