Figure 2.
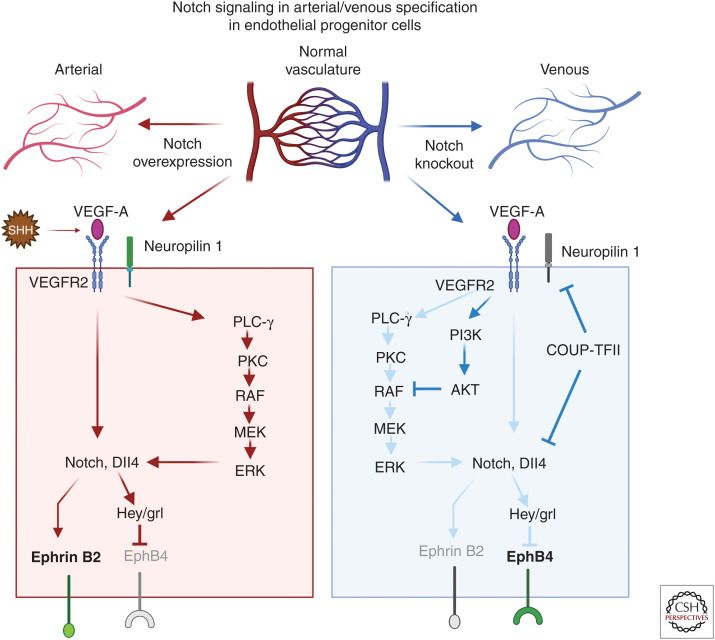
The role of Notch signaling in arterial specification during development. The top panel is a schematic showing how changes in Notch expression impact arterial/venous specification in the arterial/venous network. The two major signaling pathways operate downstream of vascular endothelial growth factor (VEGF)-A, induce arterial differentiation, the Notch pathway, and the PLC/MAPK pathway. In the arterial specification pathway (red arrows), sonic hedgehog–expressing mesodermal cells produce VEGF-A and stimulate endothelial precursor cells. Mesodermal cells expressing sonic hedgehog produce VEGF that interacts with VEGFR2 and neuropilin 1 in arterial precursor cells leading to the expression of Dll4 and Notch. Once an endothelial precursor cell reaches a critical threshold level of Dll4, it causes strong Notch signaling in an adjacent cell and establishes a cell fate decision selecting an arterial over venous endothelial cell fate. There are two distinct mechanisms that drive vein differentiation (blue arrows). The venous endothelial progenitor cells lack neuropilin 1 and low amounts of VEGF trigger expression of chicken ovalbumin upstream promoter transcription factor II (COUP-TFII). COUP-TFII represses VEGFR2, neuropilin 1, and Notch signaling and promotes a venous cell fate and promotes EphB4 expression; additionally, the activation of the PI3K/AKT pathway blocks arterial specification by preventing ERK activation. (Created in BioRender.com.)