Abstract
Background
Encephalitis is caused by autoimmune or infectious agents marked by brain inflammation. Investigations have reported altered concentrations of the cytokines in encephalitis. This study was conducted to determine the relationship between encephalitis and alterations of cytokine levels in cerebrospinal fluid (CSF) and serum.
Methods
We found possibly suitable studies by searching PubMed, Embase, Scopus, and Web of Science, systematically from inception to August 2021. 23 articles were included in the meta-analysis. To investigate sources of heterogeneity, subgroup analysis and sensitivity analysis were conducted. The protocol of the study has been registered in PROSPERO with a registration ID of CRD42021289298.
Results
A total of 23 met our eligibility criteria to be included in the meta-analysis. A total of 12 cytokines were included in the meta-analysis of CSF concentration. Moreover, 5 cytokines were also included in the serum/plasma concentration meta-analysis. According to the analyses, patients with encephalitis had higher CSF amounts of IL-6, IL-8, IL-10, CXCL10, and TNF-α than healthy controls. The alteration in the concentration of IL-2, IL-4, IL-17, CCL2, CXCL9, CXCL13, and IFN-γ was not significant. In addition, the serum/plasma levels of the TNF-α were increased in encephalitis patients, but serum/plasma concentration of the IL-6, IL-10, CXCL10, and CXCL13 remained unchanged.
Conclusions
This meta-analysis provides evidence for higher CSF concentrations of IL-6, IL-8, IL-10, CXCL10, and TNF-α in encephalitis patients compared to controls. The diagnostic and prognostic value of these cytokines and chemokines should be investigated in future studies.
1. Introduction
Encephalitis is defined as inflammation of active brain tissues that can be caused by autoimmune reactions or infections. Viral pathogens are the main culprit behind infectious encephalitis, including tick-borne encephalitis, Japanese encephalitis, and herpes simplex virus (HSV)-caused encephalitis, among others. More than 100 different pathogens and toxins have been identified as agents contributing to encephalitis pathogenesis [1]. More recently, a growing number of studies have investigated biomarkers for diagnosis and therapeutic targeting of autoimmune encephalitides, such as Anti-N-Methyl-D-Aspartate Receptor (NMDAR) [2–5].
Although the known number of etiologies for encephalitis is on the rise, it is still hard to establish the cause of encephalitis cases in clinical practice, and the underlying mechanisms are complex, not fully understood, and vary according to the cause [6–8]. According to Khetsuriani et al., From 1988 to 1997, about 18,680 cases of encephalitis were hospitalized in the United States each year; with the underlying cause for most (59.5%) not being identified [1]. Patients with encephalitis present with diverse neurological symptoms, including localizing and non-localizing symptoms such as altered mental status and seizures, alongside fever, headache, and meningeal signs [9].
Diagnosing encephalitis and its causative agents is a crucial factor in guiding treatment. A late diagnosis or an incorrect one can lead to negative clinical outcomes. The time of diagnosis might affect the prognosis of the disease vastly. Patients who are diagnosed sooner have a much better prognosis than others [10]. It is of utmost importance to have fast, reliable methods of diagnosing encephalitis in patients in order to improve the clinical outcome and prevent the patients’ conditions from getting worse. Currently, the diagnosis of encephalitis is confirmed via neuroimaging methods, mainly brain magnetic resonance imaging (MRI), and measuring cerebrospinal fluid (CSF) biomarkers of central nervous system infection and autoantibodies and interpreting electroencephalograms (EEG) in order to detect specific patterns in brain waves [11, 12]. Some studies have described increased levels of inflammatory biomarkers, such as cytokines and chemokines, in patients with encephalitis [2, 4, 13]. Cytokines and chemokines are essential agents contributing to the regulation of the innate and adaptive immune system and inflammatory reactions. Cytokines are small, low-weight polypeptides secreted by immune cells that play an important role in intercellular interactions. Different cells can secret the same cytokine, and a single cytokine can act on various cell types. They are produced and released in a cascade, leading to increased or decreased inflammatory response based on the cytokine secreted, the target tissue, and the interaction between various biochemical agents [14–18]. Different pro-inflammatory cytokines, such as interleukin (IL)-1, IL-6, IL-12, IL-18, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α and anti-inflammatory ones, such as IL-4, IL-10, IL-13, and IL-19 which are secreted from immune cells interact with body cells to mediate the immune responses in the body and thus have the most optimum inflammatory response [19]. Chemokines are a group of cytokines that are released mainly by leukocytes to induce chemotaxis in damaged tissues and attract white blood cells. In addition, chemokines could be secreted by tissue-resident cells, such as neurons and glial cells in the brain [20–22]. The term chemotaxis is used to refer to a situation in which cells adjust their movement according to the presence of specific agents in the environment. The reason might be a foreign agent such as a bacterium, fungus, virus, or simply a foreign body. The alteration in the concentration of the different chemokines, such as CXCL10, CXCL-13, CCL-4, CCL-17, CCL-20, and cytokines, including IL-2, IL-4, IL-6, IL-9, IL-10, and IFN-γ have been observed in encephalitis patients [23–30].
This study aims to systematically review studies measuring the levels of interleukins and chemokines in encephalitis, which may help identify and/or develop diagnostic signatures based on these biomarkers. Moreover, these signatures might contribute to a better understanding of the underlying pathophysiology.
2. Materials and methods
2.1. Search strategy and databases
Potentially eligible studies were found by conducting a systematic search in PubMed, Embase, Scopus, and Web of Science with no date and type of study limits. The comprehensive search string is available as S1 Table. The search has been updated until August 17th, 2021. The reference list of the retrieved studies has been screened to find further related studies. The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) has been used to report the results. The completed PRISMA checklist is available as S1 Checklist. The protocol of the study has been registered in the PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021289298) with the registration ID of CRD42021289298, and the file is available as S1 Protocol.
2.2. Selection criteria
The studies providing the concentration of the cytokines and chemokines in the plasma/serum or CSF have been considered to be eligible for the review. Studies were included if they met the following inclusion criteria: 1) original studies on human subjects, 2) diagnosis of any type of encephalitis, meningoencephalitis, and encephalomyelitis, 3) measurement of the cytokine concentration in the serum, plasma, or CSF of patients and controls (healthy controls or patients without encephalitis and other infectious diseases), and 4) adequate data for calculation of standardized mean difference (SMD). Meanwhile, the studies were excluded if they had any of the following criteria: 1) reviews, book chapters, case reports, meeting abstracts, 2) studies containing animal subjects, 3) in vivo and in vitro studies, 4) studies on gene expression of the cytokines but not their levels, 5) studies that did not have control groups 6) All cancer patients and patients with paraneoplastic encephalitis, because of the existence of cancerous tissue or remote neoplasia that might dysregulate cytokines profile, 7) concurrent complications such as pulmonary edema which could affect the concentration of cytokines, and 8) no measurement of the cytokines in the plasma, serum, or CSF. Screening and eligibility assessment were performed independently by two authors (ASK and MTPF), and discrepancies and conflicts were resolved by discussion. The third author (PS) was consulted for conflict resolution in case of disagreement.
2.3. Data extraction
Two authors have extracted the data independently (ASK and MTPF). The following data were extracted from the studies: the first author’s name, publication year, location, diagnostic criteria for encephalitis, inclusion and exclusion criteria of patients and controls, sample size, demographic information (e.g., age and sex), duration of the hospitalization, assay type, sampling sources, type of the cytokine, cytokine concentration, mean and standard deviation (SD) in patients and controls, measurement unit, and WBC levels in blood and CSF, where available. Discrepancies were resolved by discussion and agreement. When enough data was not available in the paper, we contacted the corresponding author to request further data. In case the reported concentration was median and interquartile range (IQR) or range, we used the transformation reported by Wan et al. [31], Luo et al. [32], and Shi et al. [33] to calculate mean and SD.
2.4. Quality assessment
The overall quality of the included studies was critically appraised independently by two raters (ASK and MTPF) using the Joanna Briggs Institute’s (JBI) checklist for the analytical cross-sectional studies. It has eight questions for quality assessment of the studies [34, 35]. The JBI tool assesses studies based on their inclusion criteria, study subjects and setting, measurement, confounding factors and dealing with them, outcomes, and statistical methods. The list of questions and their detailed definition is available in Table 2. The incongruences in the quality assessment were settled by consulting with the third author (PS).
Table 2. Assessment of the quality of the included studies using Joanna Briggs Institute’s (JBI) checklist.
| Study | Criteria and corresponding scores | Total | % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | |||
| Tsai, M. L. 1996 [30] | 0 | 0 | 0 | 1 | NR | NR | 1 | 1 | 3 | 37.5 |
| Singh, A. 2000 [29] | 1 | 1 | 1 | 1 | NR | NR | 1 | 1 | 6 | 75 |
| Lin, T. Y. 2002 [142] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 6 | 75 |
| Wang, S. M. 2003 [28] | 1 | 1 | 1 | 1 | NR | NR | 1 | 1 | 6 | 75 |
| Leake, J. A. 2004 [149] | 1 | 1 | 1 | 1 | NR | NR | 1 | 1 | 6 | 75 |
| Babu, G. N. 2006 [175] | 1 | 1 | 0 | 0 | NR | NR | 1 | 1 | 4 | 50 |
| Lepej, S. Z. 2007 [147] | 1 | 1 | 1 | 1 | NR | NR | 1 | 1 | 6 | 75 |
| Wang, S. M. 2008 [127] | 1 | 1 | 1 | 1 | NR | NR | 1 | 1 | 6 | 75 |
| Zajkowska, J. 2011 [179] | 1 | 1 | 1 | 1 | NR | NR | 1 | 1 | 6 | 75 |
| Ygberg, S. 2016 [125] | 1 | 1 | 1 | 1 | NR | NR | 1 | 1 | 6 | 75 |
| Singh, S. K. 2017 [131] | 1 | 1 | 1 | 0 | NR | NR | 1 | 1 | 5 | 62.5 |
| Koper, O. M. 2018 [152] | 1 | 1 | 1 | 1 | NR | NR | 1 | 1 | 6 | 75 |
| Ai, P 2018 [178] | 1 | 1 | 0 | 1 | NR | NR | 1 | 1 | 5 | 62.5 |
| Maric, L. S. 2018 [180] | 1 | 1 | 1 | 1 | NR | NR | 1 | 1 | 6 | 75 |
| Liu, B. 2018 [141] | 1 | 1 | 1 | 1 | NR | NR | 1 | 1 | 6 | 75 |
| Liu, J. 2018 [140] | 1 | 1 | 0 | 1 | NR | NR | 1 | 1 | 5 | 62.5 |
| Lin, Y. T. 2019 [25] | 1 | 1 | 1 | 1 | NR | NR | 1 | 1 | 6 | 75 |
| Peng, Y. 2019 [134] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | 100 |
| Jiang, X. Y. 2020 [156] | 1 | 1 | 0 | 1 | NR | NR | 1 | 1 | 5 | 62.5 |
| Li, J. 2020 [144] | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 7 | 87.5 |
| Ygberg, S. 2020 [126] | 1 | 1 | 1 | 1 | NR | NR | 1 | 1 | 6 | 75 |
| Zou, C. 2020 [184] | 1 | 1 | 1 | 1 | NR | NR | 1 | 1 | 6 | 75 |
| Xie, J. 2021 [183] | 1 | 1 | 0 | 1 | NR | NR | 0 | 1 | 4 | 50 |
#1: Were the criteria for inclusion in the sample clearly defined? #2: Were the study subjects and the setting described in detail? #3: Was the exposure measured in a valid and reliable way? #4: Were objective, standard criteria used for measurement of the condition? #5: Were confounding factors identified? #6: Were strategies to deal with confounding factors stated? #7: Were the outcomes measured in a valid and reliable way? #8: Was appropriate statistical analysis used?
Abbreviations: NR, not reported
0: The criterion is not fulfilled or reported by the study
1: The criterion is met by the study
2.5. Data synthesis and meta-analysis
The mean and SD of cytokine levels have been gathered as continuous data in encephalitis patients and the control group. When studies have reported concentrations of cytokines in more than one encephalitis group, if the types of encephalitis were similar (all groups had infectious encephalitis or all groups had autoimmune encephalitis), we pooled the mean and SD of the concentrations in those groups. In case median and IQR or range had been reported instead of mean and SD, they were converted to mean and SD. The between-group Hedges’ g standardized mean difference (SMD) was calculated based on sample size, mean, and SD to measure the effect size (ES) of the studies. Hedges’ g is similar to Cohen’s d with an adjustment for small samples. SMD and its 95% confidence interval (CI) were used to represent the difference in cytokines between the encephalitis group and controls. The ES of 0.2, 0.5, and 0.8 demonstrate a small, moderate, and large effect, respectively [36]. Cochran’s Q test was used to assess heterogeneity, and a P-value of 0.10 was considered as the existence of heterogeneity. Moreover, I2 was used for a more precise estimation of the heterogeneity, and I2 < 25%, 25–75%, and >75% are deemed as low, moderate, and high heterogeneity, respectively. Random effect model analysis using the DerSimonian and Laird method was used for meta-analysis, and the P-value equal to or less than 0.05 was considered significant [37]. Subgroup analysis was deployed based on whether the type of encephalitis is infectious or autoimmune. The overall effect for each subgroup is reported when at least two studies exist in the subgroup. In addition, the comparison of the subgroups was performed when each subgroup had at least two studies. Meta-regression was also performed based on the mean age of the participants of each study if it was possible (more than two studies with reported age were present in the meta-analysis; studies that did not report the mean or median age of the participants were omitted from meta-regression). Funnel plot asymmetry and Egger’s test were used for evaluation of the publication bias in the included studies. To further assess the source of the heterogeneity, sensitivity analysis was used to determine potentially influential cases. Each time one study was removed, and the effect size was recalculated to optimize the robustness of the combined effect estimate and examine its influence on the pooled SMD. All statistical analysis has been performed using the “meta” package of R software (version 4.1.1) [38].
3. Results
3.1. Study selection
The search was conducted using EMBASE, Scopus, PubMed, and Web of Science Databases for related articles, which yielded a total of 8,685 records. After searching for duplicates and removing them, 3,054 results remained for screening, of which 159 articles remained after title/abstract screening. In the process of full-text revision, 88 articles did not meet the eligibility criteria and were excluded for different reasons, as outlined in Fig 1 based on PRISMA guidelines (_((((xxx))))_)[39–103]. In some of the excluded articles, the study was conducted to compare cytokines levels in other illnesses or different stages of encephalitis, all lacking control groups (_((((xxx))))_)[44, 45, 47, 50, 53, 54, 64–66, 69, 71, 72, 74–76, 80–86, 89, 90, 94, 95, 100–103]. There were articles whose results could not be included in this review because of being in vivo, in vitro, case reports, review articles, and conference abstracts (_((((xxx))))_)[39, 40, 42, 46, 49, 51, 52, 55, 57, 59, 61, 63, 67, 68, 73, 78, 79, 93, 97–99]. In 12 studies, there were neither qualitative reports of cytokine levels nor were there quantitative data of cytokine concentrations; therefore, they did not meet our inclusion criteria [41, 48, 56, 58, 60, 62, 70, 77, 87, 88, 91, 92]. Two studies measured the efficacy and side effects of vaccines [43, 96]. The full-text article could not be found in 21 studies (_((((xxx))))_)[80, 104–123]. Based on the inclusion and exclusion criteria, 71 studies (_((((xxx))))_)[8, 23–30, 121, 124–184] were considered for this study, of which 23 studies had sufficient quantitative data and were included in the meta-analysis and reported in this study (_((((xxx))))_)[25, 28–30, 125–127, 131, 134, 140–142, 144, 147, 149, 152, 156, 175, 178–180, 183, 184]. The remaining 48 studies were not included in the meta-analysis because they mostly reported qualitative data and did not contain sufficient quantitative data. Our study does not report qualitative data and only focuses on the meta-analysis of quantitative data. Although Chen et al. [8] study investigated the concentration of cytokines, the reported data overlapped Zou et al. [184] study, published more recently, so we considered the latter in the meta-analysis. The meta-analysis was performed for 12 cytokines and chemokines, each of which was assessed in at least three comparison studies.
Fig 1. Flow diagram of literature search and study selection.
The values demonstrate the number of documents in each category.
3.2. Characteristics and quality of the included studies
The included studies’ publication time were between 1996 and 2021. CSF was used in 11 studies for evaluating the concentration of the cytokines [29, 30, 125, 126, 134, 140, 141, 149, 156, 178, 184], plasma/serum was examined for cytokines concentration in 2 studies [28, 142], and both CSF and serum/plasma were investigated in 9 studies [25, 127, 131, 144, 147, 152, 175, 179, 180, 183]. Twelve cytokines and chemokines were included in the CSF meta-analysis, namely IL-2 [149, 156, 175], IL-4 [125, 126, 156, 183], IL-6 [25, 125, 126, 134, 141, 156, 178, 184], IL-8 [29, 30, 125, 126, 140, 156], IL-10 [25, 125, 126, 134, 149, 156, 184], IL-17 [25, 125, 141, 178], TNF-α [134, 144, 156, 175, 184], IFN-γ [125, 126, 149, 156, 183], CCL2 [125, 126, 156], CXCL9 [140, 152, 156], CXCL10 [147, 156, 179, 180], and CXCL13 [25, 156, 179]. Five cytokines, including CXCL10 [127, 147, 179, 180], CXCL13 [25, 179, 180], IL-6 [25, 131, 142], IL-10 [25, 28, 131], and TNF-α [28, 142, 144, 175] were encompassed in the serum/plasma meta-analysis. The concentration of the cytokines were measured using enzyme-linked immunosorbent assay (ELISA) (_((((xxx))))_)[25, 28–30, 131, 134, 141, 142, 147, 149, 152, 175, 178–180, 183, 184], flowcytometry immunoassay [28, 127, 140], and multiplex assay [125, 126, 156]. The total number of the included subjects in the serum meta-analysis is 421, comprised of 241 patients and 180 controls. Concerning CSF meta-analysis, a total of 1062 individuals are available, including 670 patients 392 controls. The summary of the characteristics of the studies is available in the Table 1.
Table 1. Summary of findings from studies included in the quantitative analysis of serum/plasma or CSF levels in encephalitis disease.
| First Author | Year | Country | CSF/Serum | Measured Cytokine(s) | Encephalitis Type | Patients N | Controls N | Assay Type | Female % | Mean Age | Encephalitis Type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tsai, M. L. [30] | 1996 | Taiwan | CSF | IL-8 | Infectious | 11 | 11 | ELISA | - | - | |
| Singh, A. [29] | 2000 | India | CSF | IL-8 | Infectious | 30 | 27 | ELISA | - | - | JE |
| Lin, T. Y. [142] | 2002 | Taiwan | Serum | IL-6, TNF-α | Infectious | 8 | 21 | ELISA | 14.19 | 19.59 | |
| Wang, S. M. [28] | 2003 | Taiwan | Serum | IL-2, IL-4, IL-10, TNF-α, IFN-γ | Infectious | 34 | 15 | Multiplex Assay | - | - | EV71-associated BE |
| Leake, J. A. [149] | 2004 | USA | CSF | IL-2, IL-10, IFN-γ | Auto-Immune | 11 | 28 | ELISA | - | - | ADEM |
| Babu, G. N. [175] | 2006 | India | CSF & Serum | IL-2, TNF-α | Infectious | 18 | 20 | ELISA | - | - | JE |
| Lepej, S. Z. [147] | 2007 | Croatia | CSF & Serum | CXCL10 | Infectious | 19 | 10 | Immunoassay | 50.34 | 34.90 | TBE patient |
| Wang, S. M. [127] | 2008 | Taiwan | CSF & Serum | CCL2, IL-8, CXCL10 | Infectious | 21 | 13 | Multiplex Assay | - | - | EV71-associated BE |
| Zajkowska, J. [179] | 2011 | Poland | CSF & Serum | CXCL10, CXCL13 | Infectious | 15 | 8 | ELISA | 33.30 | 43.00 | TBE |
| Ygberg, S. [125] | 2016 | Sweden | CSF | IL-4, IL-6, IL-8, IL-10, IL-17, CCL2, IFN-γ | Both | 17 | 13 | Multiplex Assay | 64.68 | 51.29 | 13 anti-NMDAR + 4 Infectious |
| Singh, S. K. [131] | 2017 | India | CSF & Serum | IL-6, IL-10 | Both | 87 | 64 | ELISA | 98.00 | - | 13 AES+JE, 74 AES |
| Koper, O. M. [152] | 2018 | Poland | CSF & Serum | CXCL9 | Infectious | 24 | 13 | ELISA | 64.24 | 47.11 | TBE |
| Ai, P [178] | 2018 | China | CSF | IL-6, IL-17 | Auto-Immune | 33 | 38 | ELISA | 54.46 | 37.55 | Anti-NMDAR |
| Maric, L. S. [180] | 2018 | Croatia | CSF & Serum | CXCL10, CXCL13 | Auto-Immune | 23 | 20 | ELISA | - | - | ADEM |
| Liu, B. [141] | 2018 | China | CSF | IL-6 | Auto-Immune | 24 | 31 | ELISA | 47.28 | 36.98 | NMDAR |
| Liu, J. [140] | 2018 | China | CSF | IL-8, IL-10, CXCL9 | Infectious | 99 | 22 | Multiplex Assay | 39.67 | 28.79 | EV71-associated BE |
| Lin, Y. T. [25] | 2019 | China | CSF & Serum | IL-6, IL-10, IL-17, CXCL13 | Auto-Immune | 16 | 9 | ELISA | 15.92 | 51.65 | Anti-LGI1 encephalitis |
| Peng, Y. [134] | 2019 | China | CSF | IL-10, TNF-α | Auto-Immune | 33 | 17 | ELISA | 56.00 | 34.87 | NMDAR |
| Jiang, X. Y. [156] | 2020 | China | CSF | IL-2, IL-4, IL-6, IL-8, IL-10, TNF-α, CCL2, CXCL9, CXCL10, CXCL13, IFN-γ | Auto-Immune | 147 | 35 | Multiplex Assay | - | - | NMDAR |
| Ygberg, S. [126] | 2020 | Sweden | CSF | IL-4, IL-6, IL-8, IL-10, CCL2, IFN-γ | Infectious | 37 | 19 | Multiplex Assay | 41.04 | 95.93 | TBE |
| Zou, C. [184] | 2020 | China | CSF | IL-6, TNF-α | Both | 46 | 21 | - | 35.70 | 61.54 | 33 NMDAR + 13 Viral |
| Li, J. [144] | 2020 | China | CSF & Serum | TNF-α | Infectious | 84 | 50 | ELISA | 44.06 | 6.37 | TBE |
| Xie, J. [183] | 2021 | China | CSF & Serum | IL-4, IFN-γ | Infectious | 80 | 40 | ELISA | 52.50 | 7.83 | 40 Viral + 40 Supporative |
Abbreviations: CSF, cerebrospinal fluid; IL-8, interleukin-8; ELISA, enzyme-linked immunoassay; JE, Japanese encephalitis; TNF-a, tumor necrosis factor-a; EV-71, enterovirus-71; BE, brainstem encephalitis; ADEM, acute disseminated encephalomyelitis; TBE, tick-borne encephalitis; NMDAR, N-methyl-D-aspartate receptor; AES, acute encephalitis syndrome; LGI1, leucine-rich glioma inactivated1; IFN-γ, Interferon γ
Using the JBI quality assessment tool, most of the studies achieved a quality score of 5 (62.5) or more, two studies had a score of 4 (50%) [175, 183], and the score of one study was 3 (37.5) [30]. A summary of the quality assessment of included studies is provided in Table 2. The funnel, drapery, and sensitivity analysis forest plots of the analyses are available in S1 File.
3.3. IL-2
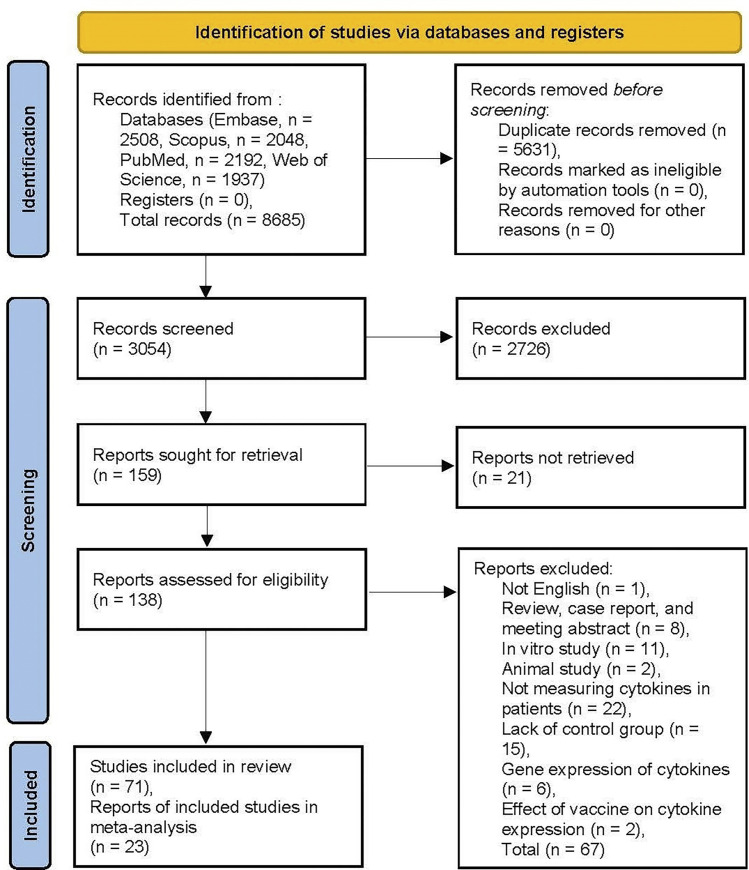
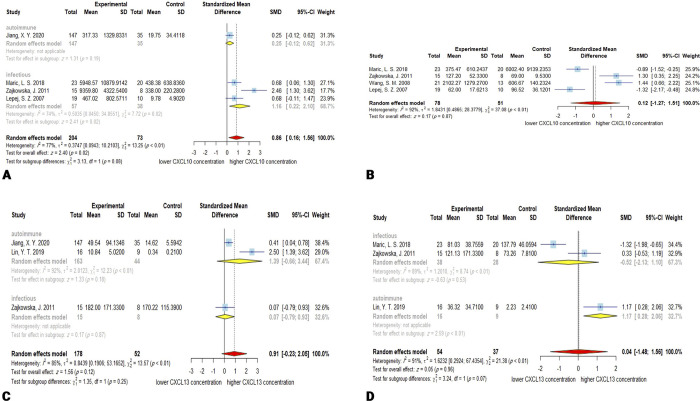
Three studies [149, 156, 175] with 176 encephalitis patients and 83 controls reported CSF concentration of IL-2. Although the overall concentration of the IL-2 was higher in encephalitis patients, this difference was not significant, with a P-value of 0.05 (SMD, 0.82; 95% CI, -0.02–1.66). The heterogeneity was considerable with Q = 11.58 (P < 0.01) and I2 = 83%. The meta-regression for age was not applicable because data for age were only available in one study. In addition, subgroup analysis demonstrated non-significant increase in the concentration of IL-2 in infectious encephalitis (SMD = 0.87; 95% CI, -0.89–2.63; P = 0.33) with high heterogeneity (Q = 11.57; P < 0.01; I2 = 91%). There was only one study available for the autoimmune subgroup; hence, the overall effect for this subgroup and comparison between subgroups are not applicable. The Egger’s test revealed no publication bias (P = 0.91). Sensitivity analysis revealed that omitting Leake, J. A. 2004 study leads the overall effect to become significant (P = 0.02) (Fig 2A).
Fig 2.
Forest plots for results of meta-analysis for IL-2 CSF concentrations (A), IL-4 CSF concentrations (B), IL-6 CSF concentrations (C), and IL-6 serum/plasma concentrations (D). SD, standard deviation; SMD, standardized mean difference; CI, confidence interval.
3.4. IL-4
241 patients and 106 controls from 4 studies [125, 126, 156, 183] were included in the meta-analysis of CSF concentration of IL-4. The meta-analysis showed slightly elevated IL-4 CSF levels, but this was not statistically significant (SMD, 1.74; 95% CI, -0.09–3.58; P = 0.06). The heterogeneity was significant with Q = 123.24 (P < 0.01) and I2 = 97%. The meta-regression demonstrated no significant effect for age as a moderator of the meta-analysis (P = 0.33). The subgroup analysis did not show significant alteration in IL-4 levels in both infectious (SMD, 2.73; 95% CI, -1.35–6.80; P = 0.19) or autoimmune encephalitis (SMD, 0.50; 95% CI, -0.89–1.90; P = 0.48). The autoimmune subgroup had less heterogeneity (Q = 4.51; P < 0.05; I2 = 78%) than the infectious group (Q = 118.72; P < 0.01; I2 = 98%). The chi-square test for assessing the disparity between subgroups demonstrated no significant difference (P = 0.31). There was no publication bias based on the Egger’s linear regression test for funnel plot asymmetry (P = 0.56). Omitting Ygberg, S. 2016 from autoimmune subgroup in the sensitivity analysis resulted in a significant overall effect (P = 0.04) (Fig 2B).
3.5. IL-6
Out of 10 studies that investigated the concentration of the IL-6, 7 reported its concentration in CSF [125, 126, 134, 141, 156, 178, 184], 2 reported its concentration in serum/plasma [131, 142], and 1 study reported both serum/plasma and CSF levels of IL-6 [25].
3.5.1. CSF IL-6
A total of 340 patients and 182 controls explored CSF concentration of IL-6. The CSF levels of the IL-6 in encephalitis patients are significantly higher than controls (SMD, 1.72; 95% CI, 0.79–2.66; P < 0.001). The overall heterogeneity is high, with Q = 129.24 (P < 0.01) and I2 = 94%. Meta-regression for age as moderator was not significant (P = 0.34). Subgroup analysis results are consistent and show that in both autoimmune and infectious encephalitis, CSF concentration of IL-6 is higher than controls (SMD (autoimmune), 1.90; 95% CI, 0.71–3.90; P < 0.01; SMD (infectious), 1.28; 95% CI, 0.75–1.80; P < 0.01). There was no significant difference between the two subgroups (P = 0.35). The tests for heterogeneity showed a high heterogeneity in the autoimmune subgroup (Q = 127.54; P < 0.01; I2 = 95%) and a lack of observed heterogeneity in the infectious subgroup (Q = 0.00; P = 0.96; I2 = 0.0%). The publication bias was not significant based on the Egger’s test (P = 0.05), and the sensitivity analysis demonstrated that Ai, P. can influence the overall effect size, without changing the statistical significance (Fig 2C).
3.5.2. Serum IL-6
Pooled data demonstrated that the serum/plasma levels of the IL-6 are not significantly higher (SMD, 0.56; 95% CI, -0.41–1.52; P = 0.26) in encephalitis patients (n = 111) than controls (n = 94). The overall heterogeneity was high (Q = 11.86; P < 0.01; I2 = 83%). Meta-regression was not applicable due to the lack of enough data. Subgroup analysis for infectious encephalitis indicated similar results to the overall pooled effect with high heterogeneity (SMD, 0.92; P = 0.28; 95% CI, -0.76–2.61; Q = 11.1, P < 0.01; I2 = 91%). Since there is only one study in the autoimmune subgroup, the overall effect for this subgroup and comparison between subgroups are not applicable. The Egger’s test showed no significant publication bias (P = 0.58). The sensitivity analysis suggested that omitting Singh, S. 2017 or Lin, Y. T. 2019 could affect the overall effect to be statistically significant. This happens because of the small number of studies and high SMD of the Lin, T. Y. 2002 study, which could affect the pooled effect in the absence of the mentioned studies (Fig 2D).
3.6. IL-8
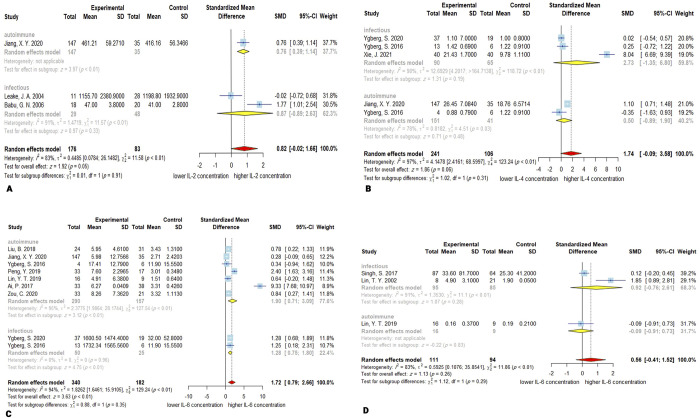
Random effect meta-analysis of 6 studies [29, 30, 125, 126, 140, 156] demonstrated that encephalitis patients (n = 341) had higher CSF IL-8 concentration compared to control group (n = 126) for IL-8 (SMD, 1.03; 95% CI, 0.17–1.90; P < 0.05). The overall heterogeneity calculated is Q = 70.63 (P < 0.01), I2 = 92%. Meta-regression demonstrated significant effect of age on the CSF concentration of IL-8 (P < 0.05). In subgroup analysis, the CSF concentration of IL-8 in the autoimmune subgroup was significantly higher than controls (SMD, 1.15; 95% CI, 0.77–1.52; P < 0.01). In infectious subgroup, IL-8 CSF levels were not statistically different from controls (SMD, 1.09; 95% CI, -0.19–2.38; P = 0.10). The most observed heterogeneity was attributable to the infectious subgroup (Q = 66; P < 0.01; I2 = 94%), and there was no considerable observed heterogeneity in autoimmune subgroup (Q = 0.79; P = 0.37; I2 = 0.0%). The Between-subgroup test did not show any significant difference (P = 0.94). The Egger’s linear regression test for meta bias reported that publication bias was not significant (P = 0.68). Sensitivity analysis indicates that removing Singh, A. 2000 and Jiang, X. Y. 2020 studies leads to a non-significant overall effect. This is possibly due to the high effect size reported in Singh, A. 2000 study and robust large sample size in Jiang, X. Y. 2020, which greatly influences the overall result (Fig 3A).
Fig 3.
Forest plots for results of meta-analysis for IL-8 CSF concentrations (A), IL-10 CSF concentration (B), IL-10 serum/plasma concentrations (C), and IL-17 CSF concentrations (D). SD, standard deviation; SMD, standardized mean difference; CI, confidence interval.
3.7. IL-10
Nine studies [25, 28, 125, 126, 131, 134, 149, 156, 184] have investigated the concentration of IL-10: 6 studies in CSF [125, 126, 134, 149, 156, 184], 2 studies in serum/plasma [28, 131], and 1 study in both [25].
3.8. CSF IL-10
The higher CSF concentration of IL-10 in encephalitis patients (n = 294) compared to controls (n = 141) was confirmed by meta-analysis (SMD, 1.31; 95% CI, 0.36–2.26; P < 0.01). The heterogeneity among the studies is significant (Q = 95.08; P < 0.0001; I2 = 93%). The meta-regression demonstrated no significant effect of the age on the CSF concentration of the IL-10 (P = 0.24). In the autoimmune subgroup the effect was consistent with the overall effect (SMD, 2.18; 95% CI, 0.60–3.77; P < 0.01), but the infectious subgroup exhibited no significant alteration of the CSF IL-10 levels (SMD, 014; 95% CI, -0.47–0.74; P = 0.66). The test for the difference between subgroups was significant (P = 0.02). The existing heterogeneity can be mostly ascribed to the autoimmune subgroup with Q = 81.74 (P < 0.01; I2 = 95), and the heterogeneity in the infectious was moderate (Q = 4.13; P = 0.13; I2 = 52%). The difference between subgroups was significant with P-value of 0.02. The Egger’s test for the publication bias was not significant (P = 0.16). The sensitivity analysis revealed no single study with significant impact of the overall effect (Fig 3B).
3.9. Serum IL-10
Similarly, the concentration of the IL-10 in serum/plasma was higher in encephalitis patients (n = 137) than controls (n = 88; SMD, 0.51; 95% CI, 0.21–0.80; P < 0.001). The between studies heterogeneity was low (Q = 2.16; P = 0.34; I2 = 7%). The meta-regression for age was not applicable since the data for age was only available in one study. There was only one study in the autoimmune subgroup, and the pooled effect and evaluation of the heterogeneity were not applicable. Serum/plasma alteration of IL-10 levels in the infectious group was not significant (SMD, 0.43; 95% CI, -0.05–0.92; P = 0.08). The heterogeneity was moderate (Q = 2.11; P = 0.15; I2 = 53%). The publication bias was not significant (P-value = 0.66). Furthermore, the sensitivity analysis demonstrated that omitting either Singh, S. 2017, or Lin, Y. T. 2019 will make the overall effect non-significant, which may be a consequence of the low number of studies available for the meta-analysis (Fig 3C).
3.10. IL-17
Four studies reported the CSF concentration of IL-17 or IL-17A for encephalitis patients (n = 90) and control group (n = 90). The overall levels of IL-17 were not significantly higher in encephalitis patients compared with controls (SMD, 0.44; 95% CI, -0.32–1.20; P = 0.26). The heterogeneity was high with Q = 19.51 (P < 0.001), and I2 = 80%. The meta-regression demonstrated a non-significant effect of age on the CSF concentration of the IL-17 (P = 0.54). The subgroup analysis demonstrated that in autoimmune subgroup IL-17 levels were not significantly altered (SMD, 0.41; 95% CI, -0.52–1.34; P = 0.38), with a high heterogeneity among studies (Q = 19.42; P < 0.01; I2 = 85%). The pooled estimates were not performable for the infectious subgroup because only one study belongs to it. The Egger’s test for funnel plot asymmetry reported no significant publication bias (P = 0.40). The sensitivity analysis demonstrated that removing Lin, Y. T. 2019, because of its reverse reported effect, increases the overall estimate and leads to significantly higher IL-17 levels in patients than controls (P < 0001) (Fig 3D).
3.11. CCL2
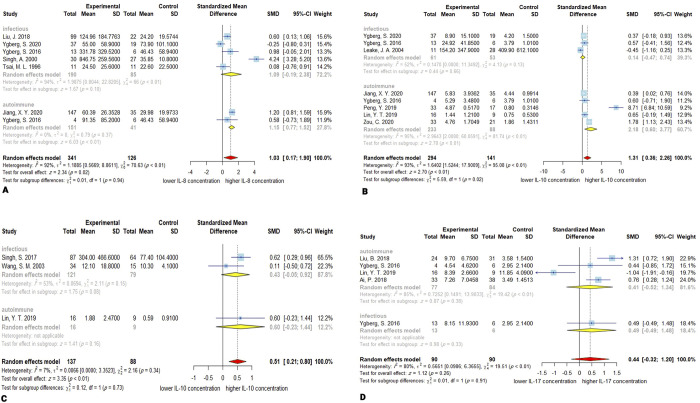
Three studies with 201 encephalitis patients and 66 controls investigated the CSF concentration of CCL2. The overall effect tended to show decreased concentration of CCL2 (SMD, -0.53; 95% CI, -1.08–0.01; P = 0.06). The between-study heterogeneity was moderate (Q = 7.11; P = 0.07; I2 = 58%). Moreover, the meta-regression demonstrated no significant moderating effect for age in the meta-analysis (P = 0.10). In the autoimmune subgroup the overall CCL2 levels were significantly decreased (SMD, -0.54; 95% CI, -0.90–-0.18; P <0.01), but this reduction was not significant in the infectious subgroup (SMD, -0.40; 95% CI, -1.89–1.10; P = 0.60). There was no difference between subgroups (P = 0.86). There was no heterogeneity in the autoimmune subgroup (Q = 0; P = 1; I2 = 0%), and the infectious subgroup is mostly responsible for the overall heterogeneity (Q = 6.85; P < 0.01; I2 = 85%). Publication bias was not significant (P = 0.70). Based on the sensitivity analysis, omitting the Ygberg, S. 2016 from infectious subgroup will result in significant overall effect size (Fig 4A).
Fig 4.
Forest plots for results of the meta-analysis for CCL2 CSF concentrations (A) and CXCL9 CSF concentrations (B). SD, standard deviation; SMD, standardized mean difference; CI, confidence interval.
3.12. CXCL9
Three studies with 270 encephalitis subjects and 70 control subjects reported the CSF concentration of the CXCL9. The overall effect shows the CSF concentration of the CXCL9 is higher in encephalitis than in control, whereas non-significant (SMD, 2.50; 95% CI, -0.15–5.16; P = 0.06). The heterogeneity is substantially high (Q = 99.75; P < 0.01; I2 = 98%). The meta-regression for age was not applicable since the data for age was only available in two studies. The subgroup analysis for the infectious encephalitis revealed significantly increased levels of CXCL9 in the CSF (SMD, 3.58; 95% CI, 3.02–4.14; P < 0.01). There was no observed heterogeneity in the infectious subgroup (Q = 0.56; P = 0.46; I2 = 0%). Egger’s linear regression for the funnel plot asymmetry exhibited no significant publication bias (P = 0.33). The estimates for the autoimmune subgroup were not applicable because there is only one study. The removal of the Jiang, X. Y. 2020 effect in the sensitivity study causes the overall effect to be significant (P < 0.0001) (Fig 4B).
3.13. CXCL10
Out of 5 studies that reported the concentration of the CXCL10, one measured CSF concentration of CXCL10 [156], one measured serum/plasma [127], and three measured both concentrations [147, 179, 180].
3.13.1. CSF CXCL10
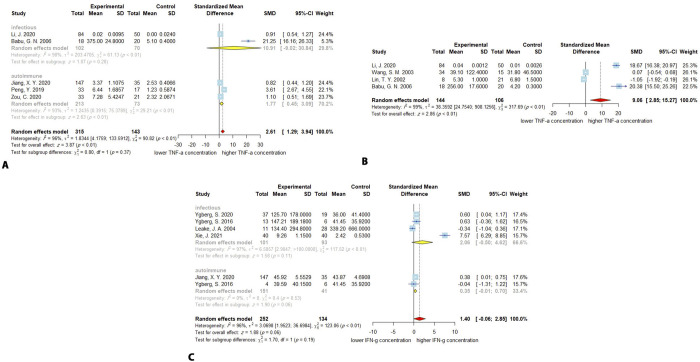
Meta-analysis of 204 encephalitis patients and 73 controls showed that the CSF levels of the CXCL10 are higher in the encephalitis group than in the control group (SMD, 0.86; 95% CI, 0.16–1.56; P = 0.02). The overall heterogeneity among studies is high (Q = 13.25; P < 0.01; I2 = 77%). The meta-regression for age as a moderator was not significant (P = 0.39). The subgroup analysis in the infectious encephalitis, similar to the overall meta-analysis, demonstrated a higher CSF concentration of the CXCL10 in the encephalitis patients compared to the controls (SMD, 1.16; 95% CI, 0.22–2.10; P = 0.02). The heterogeneity in infectious subgroup was moderate (Q = 7.72; P = 0.02; I2 = 74%). The autoimmune subgroup only had one study, and overall estimates were not applicable. Publication bias based on Egger’s linear regression test was not significant (P = 0.08). Sensitivity analysis revealed that omitting the Maric, L. S. 2018 study will cause the overall effect to become non-significant (Fig 5A).
Fig 5.
Forest plots for results of the meta-analysis for CXCL10 CSF concentrations (A), CXCL10 serum/plasma concentrations (B), CXCL13 CSF concentrations (C), and CXCL13 serum/plasma concentrations (D). SD, standard deviation; SMD, standardized mean difference; CI, confidence interval.
3.13.2. Serum CXCL10
In contrast to the CSF meta-analysis, Serum analysis reported no significant alteration in the CXCL10 concentration (npatients = 78; ncontrols = 51; SMD, 0.12; 95% CI, -1.27–1.51; P = 0.87). The observed heterogeneity was high with Q = 37.08 (P < 0.0001; I2 = 92%). The meta-regression for age as a moderator variable was not significant (P = 0.52). The subgroup analysis was not conductible since the included studies only belong to infectious encephalitis. The publication bias was not significant (P = 0.53). The sensitivity analysis revealed that omitting the studies could lead to a change in the direction of the overall effect. Except for Lepej, S. Z. 2007, whose omission leads to a slightly significant overall effect, removal of the other studies does not lead to a significant change in the overall effect (Fig 5B).
3.14. CXCL13
Among 4 studies that measured CXCL13 concentration, one investigated the CSF concentration [156], one assessed serum/plasma concentration [180], and two measured both CSF and serum/plasma concentration [25, 179].
3.14.1. CSF CXCL13
178 encephalitis patients and 52 controls entered the CSF meta-analysis for CXCL13. There was a non-statistically significant increase in the concentration of CXCL13 in patients (SMD, 091; 95% CI, -0.23–2.05; P = 0.12). The overall heterogeneity was high with Q = 13.57 (P < 0.01; I2 = 85%). The subgroup analysis in autoimmune encephalitis group demonstrated no significant alteration of the CXCL13 concentration with high between-study heterogeneity (SMD, 1.39; 95% CI, -0.66–3.44; P = 0.18; Q = 12.23; P < 0.01; I2 = 92%). The meta-regression for age was not applicable since the data for age was only available in two studies. The estimates for the infectious subgroup are not available since there was only one study. Publication bias based on Egger’s test was not significant (P = 0.59). The sensitivity analysis revealed that the removal of Lin, Y. T. 2019 could lead to a significant overall effect size with P-value = 0.04 (Fig 5C).
3.14.2. Serum CXCL13
54 cases and 37 controls were investigated for serum/plasma concentration of the CXCL13. The meta-analysis came up with no significant difference in the serum concentration of CXCL13 in encephalitis patients and controls (SMD, 0.04; 95% CI, -1.48–1.56; P = 0.96). The sample size was small, and CI was wide. The heterogeneity was high with Q = 21.38 (P < 0.0001; I2 = 91%). The meta-regression demonstrated a significant effect of age on the serum concentration of CXCL13 (P < 0.0001). The subgroup analysis results are only available for the infectious encephalitis group, which, similar to the overall effect, does not implicate any significant alteration in CXCL13 concentration (SMD, -0.52; 95% CI, -2.13–1.10; P = 0.53). The heterogeneity in infectious subgroup was also high (Q = 8.74; P < 0.01; I2 = 89%). There was no significant publication bias (P = 0.13). Sensitivity analysis reported that omitting Lin, Y. T. 2019 study leads to a slightly significant overall effect size (P = 0.04) (Fig 5D).
3.15. TNF-α
Seven studies investigated the concentration of TNF-α: two were related to serum/plasma concentration [28, 142], three to CSF concentration [134, 156, 184], and two measured both CSF and serum/plasma concentration [144, 175].
3.15.1. CSF TNF-α
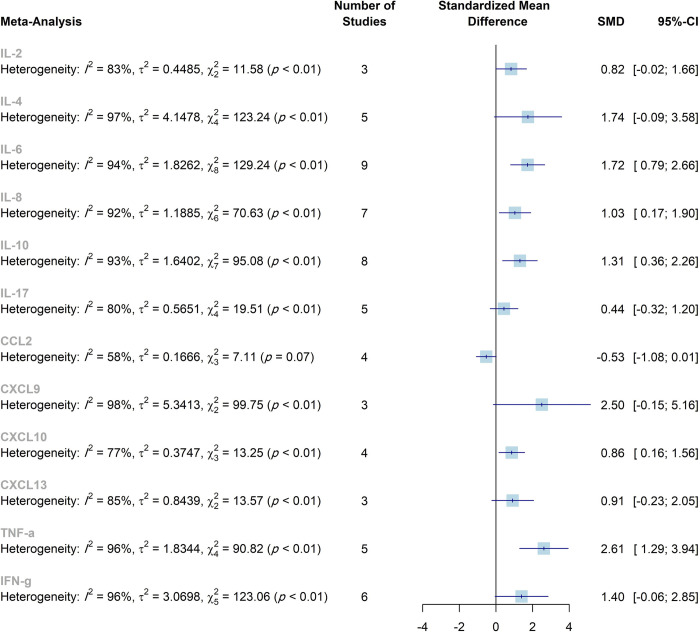
Increased CSF concentration of the TNF-α was seen in the meta-analysis of 213 encephalitis patients and 73 controls (SMD, 2.61; 95% CI, 1.29–3.94; P < 0.001). The observed heterogeneity was high with Q = 90.82 (P < 0.0001; I2 = 96%). The meta-regression demonstrated a significant effect of age on the CSF concentration of CXCL13 (P < 0.0001). The effect in the autoimmune encephalitis sub-group was similar to the overall effect (SMD, 1.77; 95% CI, 0.45–3.09; P < 0.01). Subgroup-analysis for infectious encephalitis demonstrated no significant alteration in the concentration of the TNF-α (SMD, 10.91; 95% CI, -9.02–30.84; P = 0.28). The test for subgroup difference demonstrated no significant difference (P = 0.37). The heterogeneity was high in both infectious (Q = 61.13; P < 0.01; I2 = 98%) and autoimmune (Q = 29.21; P < 0.01; I2 = 93%) subgroups. The Egger’s linear regression test for funnel plot asymmetry demonstrated significant publication bias with P-value less than 0.001. The sensitivity analysis came up with no change in the significance of the effect when putting each of studies aside (Fig 6A).
Fig 6.
Forest plots for results of the meta-analysis for TNF-α CSF concentrations (A), TNF-α serum/plasma concentrations (B), and IFN-γ CSF concentrations (C). SD, standard deviation; SMD, standardized mean difference; CI, confidence interval.
3.15.2. Serum TNF-α
The meta-analysis demonstrated that the concentration of the TNF-α is increased in the encephalitis patients compared to controls (npatients = 144; ncontrol = 106; SMD, 9.06; 95% CI, 2.85–15.27; P < 0.01). The observed heterogeneity of the reported studies was high (Q = 317.69; P < 0.0001; I2 = 99%). The meta-regression for age was not applicable since the data for was only available in two studies. The subgroup analysis was not available because all studies investigated infectious encephalitis patients. Publication bias was not significant with P-value = 0.14. The sensitivity analysis displayed that omitting each study except Li, J. 2020, which does not change the significance of the overall effect, will lead to a non-significant overall effect size (Fig 6B).
3.16. IFN-γ
252 encephalitis patients and 134 controls were included in the IFN-γ CSF concentration meta-analysis, which showed a tendency to increased concentration of IFN-γ in the former group (SMD, 1.40; 95% CI, -0.06–2.85; P = 0.06). The overall heterogeneity was high (Q = 123.06; P < 0.0001; I2 = 96%). The meta-regression demonstrated no significant effect of the age on the CSF concentration of the IFN-γ (P = 0.30). The effects in the infectious (SMD, 2.06; 95% CI, -0.50–4.62; P = 0.11) and autoimmune (SMD, 0.35; 95% CI, -0.01–0.70; P = 0.06) subgroups were consistent with the overall effect. The heterogeneity in infectious subgroups was high (Q = 117.52; P < 0.01; I2 = 97%), but the heterogeneity in autoimmune subgroup was low (Q = 0.4; P = 0.53; I2 = 0%). Publication bias was not significant with P-value = 0.34. Sensitivity analysis via leaving one study out at each time, showed that omitting Ygberg, S. 2016 and Leake, J. A. 2004 can lead to increased overall effect with a P-value of 0.046 and 0.050, respectively (Fig 6C).
4. Discussion
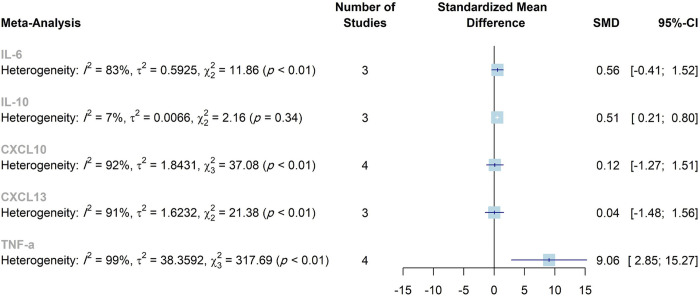
The present meta-analysis was conducted to explore cytokine levels in patients with encephalitis compared to controls without encephalitis, as well as to compare the cytokine concentrations in autoimmune encephalitis and infectious encephalitis. The cytokines IL-6, IL-8, IL-10, CXCL10, and TNF-α were significantly higher in the CSF of patients compared to controls. Also, there was a significant difference between infectious and autoimmune encephalitis regarding CSF levels of IL-10 and CXCL9. Increased serum levels of TNF-α were observed in patients with encephalitis. The Meta-analyses results have been summarized in Table 3, Figs 7 and 8, S1 and S2 Figs.
Table 3. Summary of the meta-analyses.
| Marker | Source | Subgroup | No. Studies | No. Cases | No. Controls | Meta-analysis | Heterogeneity | P-value for subgroup difference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SMD | 95% CI | P-value | I 2 | τ2 | Q | P-value1 | |||||||
| IL-2 | CSF | Autoimmune | 1 | 147 | 35 | NA | NA | NA | NA | NA | NA | NA | NA |
| Infectious | 2 | 29 | 48 | 0.87 | -0.89–2.63 | 0.33 | 91% | 1.47 | 11.57 | <0.01 | |||
| Overall | 3 | 176 | 83 | 0.82 | -0.02–1.66 | 0.05 | 83% | 0.45 | 11.58 | <0.01 | |||
| IL-4 | CSF | Autoimmune | 2 | 151 | 41 | 0.50 | -0.89–1.90 | 0.48 | 78% | 0.82 | 4.51 | <0.01 | 0.31 |
| Infectious | 3 | 90 | 65 | 2.73 | -1.35–6.80 | 0.19 | 98% | 12.69 | 118.72 | <0.01 | |||
| Overall | 5 | 241 | 106 | 1.74 | -0.09–3.58 | 0.06 | 97% | 4.15 | 123.24 | <0.01 | |||
| IL-6 | CSF | Autoimmune | 7 | 290 | 157 | 1.90 | 0.71–3.09 | <0.01 | 95% | 2.38 | 127.54 | <0.01 | <0.01 |
| Infectious | 2 | 50 | 25 | 1.28 | 0.75–1.80 | <0.01 | 0% | 0 | 0 | 0.96 | |||
| Overall | 9 | 340 | 182 | 1.72 | 0.79–2.66 | <0.01 | 94% | 1.83 | 129.24 | <0.01 | |||
| Serum | Autoimmune | 1 | 16 | 9 | NA | NA | NA | NA | NA | NA | NA | NA | |
| Infectious | 2 | 95 | 85 | 0.92 | -0.75–2.61 | 0.28 | 91% | 1.35 | 11.1 | <0.01 | |||
| Overall | 3 | 111 | 94 | 0.56 | -0.41–1.52 | 0.26 | 83% | 0.59 | 11.86 | <0.01 | |||
| IL-8 | CSF | Autoimmune | 2 | 151 | 41 | 1.15 | 0.77–1.52 | <0.01 | 0 | 0 | 0.79 | 0.37 | 0.94 |
| Infectious | 5 | 190 | 85 | 1.09 | -0.19–2.38 | 0.10 | 94% | 1.99 | 66 | <0.01 | |||
| Overall | 7 | 341 | 126 | 1.03 | 0.17–1.90 | 0.02 | 92% | 1.19 | 70.63 | <0.01 | |||
| IL-10 | CSF | Autoimmune | 5 | 233 | 88 | 2.18 | 0.60–3.77 | <0.01 | 95% | 2.96 | 81.74 | <0.01 | 0.02 |
| Infectious | 3 | 61 | 53 | 0.14 | -0.47–0.74 | 0.66 | 52% | 0.14 | 4.13 | 0.13 | |||
| Overall | 8 | 294 | 141 | 1.31 | 0.36–2.26 | <0.01 | 93% | 1.64 | 95.08 | <0.01 | |||
| Serum | Autoimmune | 1 | 16 | 9 | NA | NA | NA | NA | NA | NA | NA | NA | |
| Infectious | 2 | 121 | 79 | 0.43 | -0.05–0.92 | 0.08 | 53% | 0.07 | 2.11 | 0.15 | |||
| Overall | 3 | 137 | 88 | 0.51 | 0.21–0.80 | <0.01 | 7% | 0.01 | 2.16 | 0.34 | |||
| IL-17 | CSF | Autoimmune | 4 | 77 | 84 | 0.41 | -0.52–1.34 | 0.38 | 85% | 0.73 | 19.42 | <0.01 | NA |
| Infectious | 1 | 13 | 6 | NA | NA | NA | NA | NA | NA | NA | |||
| Overall | 5 | 90 | 90 | 0.44 | -0.32–1.20 | 0.26 | 80% | 0.47 | 19.51 | <0.01 | |||
| CCL2 | CSF | Autoimmune | 2 | 151 | 41 | -0.54 | -0.90–-0.18 | <0.01 | 0 | 0 | 0 | 1 | 0.86 |
| Infectious | 2 | 50 | 25 | -0.40 | -1.89–1.10 | 0.60 | 85% | 1.00 | 6.85 | <0.01 | |||
| Overall | 4 | 201 | 66 | -0.53 | -1.08–0.01 | 0.06 | 58% | 0.17 | 7.11 | 0.07 | |||
| CXCL9 | CSF | Autoimmune | 1 | 147 | 35 | NA | NA | NA | NA | NA | NA | NA | NA |
| Infectious | 2 | 123 | 35 | 3.58 | 3.02–4.14 | <0.01 | 0 | 0 | 0.56 | 0.46 | |||
| Overall | 3 | 270 | 70 | 2.50 | -0.15–5.16 | 0.06 | 98% | 5.34 | 99.75 | <0.01 | |||
| CXCL10 | CSF | Autoimmune | 1 | 147 | 35 | NA | NA | NA | NA | NA | NA | NA | NA |
| Infectious | 3 | 57 | 38 | 1.16 | 0.22–2.10 | 0.02 | 74% | 0.50 | 7.72 | 0.02 | |||
| Overall | 4 | 204 | 73 | 0.86 | 0.16–1.56 | 0.02 | 77% | 0.37 | 13.25 | <0.01 | |||
| CXCL10 | Serum | Infectious | 4 | 78 | 51 | 0.12 | -1.27–1.51 | 0.87 | 92% | 1.84 | 37.08 | <0.01 | NA |
| CXCL13 | CSF | Autoimmune | 2 | 163 | 44 | 1.39 | -0.66–3.44 | 0.18 | 92% | 2.01 | 12.23 | <0.01 | NA |
| Infectious | 1 | 15 | 8 | NA | NA | NA | NA | NA | NA | NA | |||
| Overall | 3 | 178 | 52 | 0.91 | -0.23–2.05 | 0.12 | 85% | 0.84 | 13.57 | <0.01 | |||
| CXCL13 | Serum | Autoimmune | 1 | 16 | 9 | NA | NA | NA | NA | NA | NA | NA | NA |
| Infectious | 2 | 38 | 28 | -0.52 | -2.13–1.10 | 0.53 | 89% | 1.20 | 8.74 | <0.01 | |||
| Overall | 3 | 54 | 37 | 0.04 | -1.48–1.56 | 0.96 | 91% | 1.62 | 21.38 | <0.01 | |||
| TNF-α | CSF | Autoimmune | 3 | 213 | 73 | 1.77 | 0.45–3.09 | <0.01 | 93% | 1.24 | 29.21 | <0.01 | 0.37 |
| Infectious | 2 | 102 | 70 | 10.91 | -9.02–30.84 | 0.28 | 98% | 203.47 | 61.13 | <0.01 | |||
| Overall | 5 | 315 | 143 | 2.61 | 1.21–3.94 | <0.01 | 96% | 1.83 | 90.82 | <0.01 | |||
| Serum | Infectious | 4 | 144 | 106 | 9.06 | 2.85–15.27 | <0.01 | 99% | 38.36 | 317.69 | <0.01 | NA | |
| IFN-γ | CSF | Autoimmune | 2 | 151 | 41 | 0.35 | -0.01–0.70 | 0.06 | 0 | 0 | 0.4 | 0.53 | 0.19 |
| Infectious | 4 | 101 | 93 | 2.06 | -0.50–4.62 | 0.11 | 97% | 6.59 | 117.52 | <0.01 | |||
| Overall | 6 | 252 | 134 | 1.40 | -0.06–2.85 | 0.06 | 96% | 3.07 | 123.06 | <0.01 | |||
1 P-value for Cochrane Q test
Abbreviations: CSF, cerebrospinal fluid; NA, not applicable
Fig 7. Summary of the overall effects and heterogeneity of the concentration of the cytokines in the CSF.
SMD, standardized mean difference; CI, confidence interval.
Fig 8. Summary of the overall effects and heterogeneity of the concentrations of the cytokines in the serum/plasma.
SMD, standardized mean difference; CI, confidence interval.
Encephalitis is a condition that can be the result of various causes. It can be classified into infectious and autoimmune encephalitis. Infectious encephalitis is caused when an infectious agent enters the body, mainly viruses, but some cases of primary encephalitis are caused by bacteria, fungi, or parasites. The common viral causes of encephalitis include HSV, JEV, varicella zoster, measles, mumps, rabies, and rubella [185–187].
Herpes simplex encephalitis (HSE) is one of the most common types of infectious encephalitis. It is caused by the HSV-1 virus–a neurotropic virus that infects the trigeminal nerve of humans [188]. Despite its widespread prevalence, it is still unknown whether HSE is caused by viral reactivation inside the trigeminal ganglia, direct primary infection of the olfactory mucosa, or other infected central nervous system (CNS) neurons in the brain [189]. Prior investigations have demonstrated that cytokines, such as TNF-α, IFN-γ, and IL-1, play an important role in the pathogenesis of HSE [190, 191].
When the body is infected with HSV, toll-like receptors (TLRs) such as TLR2, TLR3, TLR7, and TLR9 help identify the virus and then, through signaling cascades, trigger intracellular responses. Consequently, these responses stimulate the activation and migration of immune cells [191, 192]. Moreover, the activation of TLRs via HSV-1 proteins or nucleic acids can result in the expression of chemokines and pro-inflammatory cytokines that accelerate the activation of innate immune reactions [93, 193, 194]. The immune reactions stimulate numerous types of immune cells to produce a wide range of cytokines and chemokines (CXCL9), including interleukins, TNF-α, and interferon regulatory factors (IRF3, IRF7, and type I IFNs) [190, 191]. Type I interferons (IFN-α and IFN-β) are major contributors to combat viral encephalitis, including HSE. Non-immune cells also produce interferons in response to pathogens to regulate the immune system’s activity. Studies have proven that overexpression of these cytokines or chemokines could exacerbate brain damage over time. In summary, when HSV-1 infects neurons, innate immunity through IFN signaling prevents the virus from spreading [195].
Adaptive immune responses to HSV-1 and HSV-2 are complicated, involving a variety of factors, including nuclear factor kappa B (NFκB) and Janus kinase (JAK)/signal transducer and activator of transcription (STAT) [196]. Interferons could activate IFN stimulated genes (ISGs) that produce antiviral proteins in infected and surrounding cells [196]. The binding of interferons to the IFNα/β receptor activates the JAK-STAT pathway in adjacent cells. This event inhibits viral replication [197]. The innate immune response against HSV is facilitated by plasmacytoid dendritic cells (pDCs) and Natural Killer (NK) cells [196, 198]. Besides interferon, it has been demonstrated that TLR signaling could trigger the expression of proinflammatory cytokines, including pro–IL-1 β leading to neural death [199].
Wang et al. [200] reported that when comparing the expression of cytokines/chemokines in brain tissues from the experimental and control groups, 13 out of 62 components in the array were found to be differently expressed in the experimental group. Notably, all of the differentially expressed proteins (DEPs) were upregulated in the experimental group and belonged to a variety of cytokine families, including the interleukin family (IL-1, IL-2, IL-12f, IL-4, and IL-6), the CCL series (CCL-5, Macrophage inflammatory protein-1 (MIP-1 or CCL-3), and Monocyte chemoattractant protein-1 (MCP-1 or CCL-2)), the CXCL family (keratinocyte-derived cytokine), and the tumor necrosis factor family (TNF-α and soluble tumor necrosis factor receptor-Ι (sTNF-RΙ)). They mentioned that the majority of DEPs were inflammatory proteins that performed important roles in the recruitment and activation of inflammatory cells. It had not been previously documented that the cytokines IL-1, MIP-1, and sTNF-RΙ were upregulated in HSE [200]. It is generally established that IL-1, regulated upon activation, normal T cells expressed and secreted (RANTES or CCL-5), and KC stimulate the accumulation of inflammatory cells in the affected tissues. MIP-1 and MCP-1 are also known to stimulate the formation of monocyte/macrophage cell lines, whereas IL-2, TNF-α, and IL-12 are known to promote the activation and differentiation of T cells. Furthermore, IL-4 and IL-6 increase B cell activation and differentiation [201, 202]. These elements could also trigger the expression of additional cytokines and probably have a critical role in increasing inflammation and brain tissue damage in acute viral meningitis [203, 204].
The Japanese Encephalitis Virus (JEV) is the most prevalent cause of viral encephalitis in Southeast Asia, transmitted by mosquitoes. After JEV has entered the bloodstream, the viral replication peaks without any cell death or TNF-α release; then, monocytes become activated and differentiate into monocyte-derived dendritic cells (MDDCs) and monocyte-derived macrophages (MDMs) [205]. Pro-inflammatory cytokines such as TNF-α, IL-12, and IL-6 are produced by DCs infected with JEV; however, IL-10 is not produced by macrophages infected with JEV [206]. The microglial cells, which are CNS resident macrophages, could also be infected by JEV. Microglial cells play a critical role in the CNS during the JEV infection because they appear as a virus reservoir [207]. Activated microglia produce pro-inflammatory cytokines, namely TNF-α and IL-6, resulting in neuronal cell death [208]. Moreover, the study of Winter et al. [100], which was a large investigation into cytokines and chemokines for any acute viral encephalitis both in terms of the number of patients and number of investigated parameters, has demonstrated that an unregulated pro-inflammatory response in flavivirus encephalitis can be detrimental. TNF-α, IL-8, and IFN-α have been found to be related to poor outcomes in prior investigations of Japanese encephalitis (JE) patients [29, 54, 209]. In Winter et al.’s study [100], it has been established that TNF-α, IL-8, and IFN-α are critical in the development of JE, as well as the importance of IL-6 and RANTES. In addition, the West Nile virus and other flaviviruses have been shown to harm the CNS in a variety of animal models [210, 211].
Non-infectious, immune-mediated inflammation of the brain parenchyma is called autoimmune encephalitis. It can affect the cortex or deep gray matter, as well as white matter, the meninges, and the spinal cord [212–215]. Cytokines and chemokines in autoimmune encephalitis may give insight into the pathophysiology of autoimmune encephalitis. Studies in patients with NMDAR antibody-associated autoimmune encephalitis have shown an increase in serum IL-2 [172] and CSF IL-6, IL-17, CXCL-10, and IL-1β. Both innate and adaptive immunity, specifically Th1 and Th17, are involved in the secretion of these cytokines [13, 42, 151, 172, 216].
Monocytes and microglia have been found to produce CXCL13 [146]. it is a B cell chemoattractant that was found in the CSF of NMDAR antibody-associated autoimmune encephalitis patients. A decrease in CXCL13 levels was associated with a positive outcome after therapy [146]. The levels of BAFF and APRIL in the CSF of autoimmune encephalitis patients were linked to functional results in a study. According to the opposite findings, no increase in CSF levels of BAFF or APRIL was seen in research comparing NMDAR antibody-associated autoimmune encephalitis with viral encephalitis [217, 218].
Interferon-γ, IL-17, IL-12, and IL-23 levels in the CSF of autoimmune encephalitis patients with antibodies to cell surface proteins are greater than those of autoimmune encephalitis patients with antibodies to intracellular antigens [129]. According to recently published research, patients with NMDAR antibody-associated autoimmune encephalitis had increased IL-6, pentraxin-3, CD40L, and IL-17A in their CSF [141]. Patients with new-onset refractory status epilepticus who had autoimmune epilepsy were studied in a study. It was discovered that the CSF had higher IL-6, TNF-α, IL-2, and IL-4 and elevated levels of IL-6 and TNF-α in the peripheral tissues were observed [219]. Eighty-six percent of patients had improvement in seizure activity and a return to normal cytokine levels after receiving therapy with a monoclonal antibody targeting the IL-6 receptor (Tocilizumab) [219].
Our meta-analysis has several limitations. Some of the included cytokines lacked enough original studies to perform the meta-analysis. Despite performing subgroup analysis for the types of encephalitis (infectious or autoimmune), The etiologies of the disease, which may affect the concentration of cytokines in CSF or serum, were not included in the meta-analysis due to insufficient data. Moreover, due to inadequate data, the meta-regression for age, as a possible moderator variable, was not applicable in all cytokines and chemokines. Also, the sample size of most studies was small. Regarding the measurement methods, the studies used different methods and kits, which resulted in heterogeneity. Additionally, the heterogeneity was high in most of the meta-analyses. Sensitivity analysis was conducted to investigate the probable sources of heterogeneity, which remained high in some instances. To the best of our knowledge, this is the first meta-analysis investigating the serum and CSF concentrations of the cytokines in encephalitis. Also, the levels of the cytokines have been compared between autoimmune and infectious encephalitis in subgroup analysis.
5. Conclusions
This meta-analysis serves as evidence that encephalitis patients had greater CSF concentrations of IL-6, IL-8, IL-10, CXCL10, and TNF-α when compared to controls. TNF-α also showed increased concentration in serum. Moreover, it was observed that IL-10 had higher levels in autoimmune encephalitis compared to autoimmune encephalitis. Contrastingly, CXCL9 had a higher concentration in infectious encephalitis. Accordingly, the interleukin antagonists could be investigated as a potential adjunctive treatment in encephalitis. The diagnostic and prognostic sensitivity and specificity of these cytokines may also be evaluated in future studies. Furthermore, the prospect of antiviral medication and other viable treatment methods needs much more extensive exploration to avoid disease progression and the frequently severe sequalae after encephalitis infection.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(TIF)
(TIF)
Abbreviations
- NMDAR
N-Methyl-D-Aspartate Receptor
- MRI
magnetic resonance imaging
- CSF
cerebrospinal fluid
- EEG
electroencephalograms
- IL-1
Interleukin-1
- EEG
electroencephalogram
- IL-1
interleukin-1
- IFN-γ
interferon-γ
- TNF-α
tumor necrosis factor-α
- SD
standard deviation
- IQR
interquartile range
- JBI
Joanna Briggs Institute’s
- ES
effect size
- CI
confidence interval
- ELISA
enzyme-linked immunosorbent assay
- HSV-1
Herpes simplex virus
- HSE
herpes simplex encephalitis
- CNS
central nervous system
- PRR
pattern recognition receptor
- TLR
toll-like receptors
- IRF3
interferon regulatory factors
- pDC
plasmacytoid dendritic cells
- NK
Natural Killer
- PAMP
pathogen-associated molecular patterns
- DEP
differentially expressed proteins
- MIP-1
Macrophage inflammatory protein
- sTNF-RΙ
soluble tumor necrosis factor receptor-Ι
- MDDC
monocyte-derived dendritic cell
- JE
Japanese encephalitis
- BAFF
B Cell Activating Factor
- APRIL
A proliferation-inducing ligand
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Khetsuriani N, Holman RC, Anderson LJ. Burden of encephalitis-associated hospitalizations in the United States, 1988–1997. Clinical Infectious Diseases. 2002;35(2):175–82. doi: 10.1086/341301 [DOI] [PubMed] [Google Scholar]
- 2.Ciano-Petersen NL, Cabezudo-García P, Muñiz-Castrillo S, Honnorat J, Serrano-Castro PJ, Oliver-Martos B. Current Status of Biomarkers in Anti-N-Methyl-D-Aspartate Receptor Encephalitis. Int J Mol Sci. 2021;22(23). doi: 10.3390/ijms222313127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gong X, Luo R, Liu J, Guo K, Li A, Liu X, et al. Efficacy and tolerability of intravenous immunoglobulin versus intravenous methylprednisolone treatment in anti-N-methyl-d-aspartate receptor encephalitis. Eur J Neurol. 2021. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Ma L, Ma X, Ma X, Li J, Li D, et al. Simple and effective serum biomarkers potential for predicting status epilepticus in anti-N-methyl-D-aspartate receptor encephalitis. BMC Neurol. 2022;22(1):27. doi: 10.1186/s12883-021-02545-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma X, Lu Y, Peng F, Wang Y, Sun X, Luo W, et al. Serum NfL associated with anti-NMDA receptor encephalitis. Neurol Sci. 2022. doi: 10.1007/s10072-021-05838-3 [DOI] [PubMed] [Google Scholar]
- 6.Granerod J, Tam CC, Crowcroft NS, Davies NW, Borchert M, Thomas SL. Challenge of the unknown. A systematic review of acute encephalitis in non-outbreak situations. Neurology. 2010;75(10):924–32. doi: 10.1212/WNL.0b013e3181f11d65 [DOI] [PubMed] [Google Scholar]
- 7.Halperin JJ. Diagnosis and management of acute encephalitis. Handb Clin Neurol. 2017;140:337–47. doi: 10.1016/B978-0-444-63600-3.00018-0 [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Ding Y, Zheng D, Wang Z, Pan S, Ji T, et al. Elevation of YKL-40 in the CSF of anti-NMDAR encephalitis patients is associated with poor prognosis. Frontiers in Neurology. 2018;9(OCT). doi: 10.3389/fneur.2018.00727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutra LA, Abrantes F, Toso FF, Pedroso JL, Barsottini OGP, Hoftberger R. Autoimmune encephalitis: a review of diagnosis and treatment. Arq Neuropsiquiatr. 2018;76(1):41–9. doi: 10.1590/0004-282X20170176 [DOI] [PubMed] [Google Scholar]
- 10.Feng S, Chen JX, Zheng P, Zhang JZ, Gao ZJ, Mao YY, et al. Status epilepticus associated with Mycoplasma pneumoniae encephalitis in children: good prognosis following early diagnosis and treatment. Chin Med J (Engl). 2019;132(12):1494–6. doi: 10.1097/CM9.0000000000000233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacchi S, Franke K, Wewegama D, Needham E, Patel S, Menon D. Magnetic resonance imaging and positron emission tomography in anti-NMDA receptor encephalitis: A systematic review. J Clin Neurosci. 2018;52:54–9. doi: 10.1016/j.jocn.2018.03.026 [DOI] [PubMed] [Google Scholar]
- 12.Hermetter C, Fazekas F, Hochmeister S. Systematic Review: Syndromes, Early Diagnosis, and Treatment in Autoimmune Encephalitis. Front Neurol. 2018;9:706. doi: 10.3389/fneur.2018.00706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kothur K, Wienholt L, Brilot F, Dale RC. CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: A systematic review. Cytokine. 2016;77:227–37. doi: 10.1016/j.cyto.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 14.Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45(2):27–37. doi: 10.1097/AIA.0b013e318034194e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang JM. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience. 2006;142(3):809–22. doi: 10.1016/j.neuroscience.2006.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLeo JA, Colburn RW, Nichols M, Malhotra A. Interleukin-6-mediated hyperalgesia/allodynia and increased spinal IL-6 expression in a rat mononeuropathy model. J Interferon Cytokine Res. 1996;16(9):695–700. doi: 10.1089/jir.1996.16.695 [DOI] [PubMed] [Google Scholar]
- 17.Schäfers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23(7):2517–21. doi: 10.1523/JNEUROSCI.23-07-02517.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heijmans-Antonissen C, Wesseldijk F, Munnikes RJ, Huygen FJ, van der Meijden P, Hop WC, et al. Multiplex bead array assay for detection of 25 soluble cytokines in blister fluid of patients with complex regional pain syndrome type 1. Mediators Inflamm. 2006;2006(1):28398. doi: 10.1155/MI/2006/28398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117(4):1162–72. doi: 10.1378/chest.117.4.1162 [DOI] [PubMed] [Google Scholar]
- 20.Mélik-Parsadaniantz S, Rostène W. Chemokines and neuromodulation. J Neuroimmunol. 2008;198(1):62–8. doi: 10.1016/j.jneuroim.2008.04.022 [DOI] [PubMed] [Google Scholar]
- 21.Ubogu EE, Cossoy MB, Ransohoff RM. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci. 2006;27(1):48–55. doi: 10.1016/j.tips.2005.11.002 [DOI] [PubMed] [Google Scholar]
- 22.Jaerve A, Müller HW. Chemokines in CNS injury and repair. Cell Tissue Res. 2012;349(1):229–48. doi: 10.1007/s00441-012-1427-3 [DOI] [PubMed] [Google Scholar]
- 23.Toczylowski K, Grygorczuk S, Osada J, Wojtkowska M, Bojkiewicz E, Wozinska-Klepadlo M, et al. Evaluation of cerebrospinal fluid CXCL13 concentrations and lymphocyte subsets in tick-borne encephalitis. Int J Infect Dis. 2020;93:40–7. doi: 10.1016/j.ijid.2020.01.023 [DOI] [PubMed] [Google Scholar]
- 24.Guziejko K, Czupryna P, Pancewicz S, Swierzbinska R, Dunaj J, Kruszewska E, et al. Analysis of CCL-4, CCL-17, CCL-20 and IL-8 concentrations in the serum of patients with tick-borne encephalitis and anaplasmosis. Cytokine. 2020;125:9. doi: 10.1016/j.cyto.2019.154852 [DOI] [PubMed] [Google Scholar]
- 25.Lin YT, Yang X, Lv JW, Liu XW, Wang SJ. CXCL13 Is A Biomarker Of Anti-Leucine-Rich Glioma-Inactivated Protein 1 Encephalitis Patients. Neuropsychiatr Dis Treat. 2019;15:2909–15. doi: 10.2147/NDT.S222258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamai M, Kobayashi N, Shimada K, Oka N, Takahashi M, Tanuma A, et al. Increased interleukin-1β and basic fibroblast growth factor levels in the cerebrospinal fluid during human herpesvirus-6B (HHV-6B) encephalitis. Biochem Biophys Res Commun. 2017;486(3):706–11. doi: 10.1016/j.bbrc.2017.03.102 [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Li W, Yang X, Zhang T, Wang Y, Zhong R, et al. Interleukin-8 is elevated in severe hand, foot, and mouth disease. Journal of Infection in Developing Countries. 2014;8(1):94–100. doi: 10.3855/jidc.3542 [DOI] [PubMed] [Google Scholar]
- 28.Wang SM, Lei HY, Huang KJ, Wu JM, Wang JR, Yu CK, et al. Pathogenesis of enterovirus 71 brainstem encephalitis in pediatric patients: Roles of cytokines and cellular immune activation in patients with pulmonary edema. J Infect Dis. 2003;188(4):564–70. doi: 10.1086/376998 [DOI] [PubMed] [Google Scholar]
- 29.Singh A, Kulshreshtha R, Mathur A. Secretion of the chemokine interleukin-8 during Japanese encephalitis virus infection. J Med Microbiol. 2000;49(7):607–12. doi: 10.1099/0022-1317-49-7-607 [DOI] [PubMed] [Google Scholar]
- 30.Tsai ML, Chen WC, Wang YC, Hung KL. Cerebrospinal fluid interleukin-6, interleukin-8, and tumor necrosis factor-alpha in children with central nervous system infections. Zhonghua Minguo xiao er ke yi xue hui za zhi [Journal] Zhonghua Minguo xiao er ke yi xue hui. 1996;37(1):16–21. [PubMed] [Google Scholar]
- 31.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology. 2014;14(1):135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–805. doi: 10.1177/0962280216669183 [DOI] [PubMed] [Google Scholar]
- 33.Shi J, Luo D, Weng H, Zeng X-T, Lin L, Chu H, et al. Optimally estimating the sample standard deviation from the five-number summary. Research Synthesis Methods. 2020;11(5):641–54. doi: 10.1002/jrsm.1429 [DOI] [PubMed] [Google Scholar]
- 34.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. JBI manual for evidence synthesis. Adelaide, Australia: JBI. 2020:255–7. [Google Scholar]
- 35.Ma L-L, Wang Y-Y, Yang Z-H, Huang D, Weng H, Zeng X-T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Military Medical Research. 2020;7(1):7. doi: 10.1186/s40779-020-00238-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions: John Wiley & Sons; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 38.Schwarzer G. meta: An R package for meta-analysis. R news. 2007;7(3):40–5. [Google Scholar]
- 39.Zhao ML, Kim MO, Morgello S, Lee SC. Expression of inducible nitric oxide synthase, interleukin-1 and caspase-1 in HIV-1 encephalitis. J Neuroimmunol. 2001;115(1–2):182–91. doi: 10.1016/s0165-5728(00)00463-x [DOI] [PubMed] [Google Scholar]
- 40.Zhang WJ, Huang ZG, Huang MY, Zeng JC. Predicting Severe Enterovirus 71-Infected Hand, Foot, and Mouth Disease: Cytokines and Chemokines. Mediat Inflamm. 2020;2020:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang GY, Wang JW, Yao G, Shi BH. Downregulation of CCL2 induced by the upregulation of microRNA-206 is associated with the severity of HEV71 encephalitis. Mol Med Rep. 2017;16(4):4620–6. doi: 10.3892/mmr.2017.7142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng C, Chen L, Chen B, Cai Y, Li P, Yan L, et al. Th17 cells were recruited and accumulated in the cerebrospinal fluid and correlated with the poor prognosis of anti-NMDAR encephalitis. Acta biochimica et biophysica Sinica. 2018;50(12):1266–73. doi: 10.1093/abbs/gmy137 [DOI] [PubMed] [Google Scholar]
- 43.Yao YF, Xu XW, Li YH, Wang XN, Yang HJ, Chen J, et al. Study of the association of seventeen single nucleotide polymorphisms and their haplotypes in the TNF-alpha, IL-2, IL-4 and IL-10 genes with the antibody response to inactivated Japanese encephalitis vaccine. Human Vaccines Immunother.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu Y, Li S, Cai C, Liu J, Wang Y, Jiang Y, et al. Characterization of inflammatory cytokine profiles in cerebrospinal fluid of hand, foot, and mouth disease children with enterovirus 71-related encephalitis in Hangzhou, Zhejiang, China. Medicine (United States). 2019;98(52). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang SM, Lei HY, Huang MC, Su LY, Lin HC, Yu CK, et al. Modulation of cytokine production by intravenous immunoglobulin in patients with enterovirus 71-associated brainstem encephalitis. Journal of Clinical Virology. 2006;37(1):47–52. doi: 10.1016/j.jcv.2006.05.009 [DOI] [PubMed] [Google Scholar]
- 46.Wang GF, Li WZ, Li KS. Acute encephalopathy and encephalitis caused by influenza virus infection. Curr Opin Neurol. 2010;23(3):305–11. doi: 10.1097/wco.0b013e328338f6c9 [DOI] [PubMed] [Google Scholar]
- 47.Torre D, Zeroli C, Ferrario G, Pugliese A, Speranza F, Orani A, et al. Levels of nitric oxide, gamma interferon and interleukin-12 in AIDS patients with toxoplasmic encephalitis. Infection. 1999;27(3):218–20. doi: 10.1007/BF02561533 [DOI] [PubMed] [Google Scholar]
- 48.Toporkova MG, Aleshin SE, Ozherelkov SV, Nadezhdina MV, Stephenson JR, Timofeev AV. Serum levels of interleukin 6 in recently hospitalized tick-borne encephalitis patients correlate with age, but not with disease outcome. Clin Exp Immunol. 2008;152(3):517–21. doi: 10.1111/j.1365-2249.2008.03617.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solanki A, Radotra BD, Vasishta RK. Correlation of cytokine expression with rabies virus distribution in rabies encephalitis. J Neuroimmunol. 2009;217(1–2):85–9. doi: 10.1016/j.jneuroim.2009.09.019 [DOI] [PubMed] [Google Scholar]
- 50.Schuster FL, Yagi S, Gavali S, Michelson D, Raghavan R, Blomquist I, et al. Under the radar: Balamuthia amebic encephalitis. Clinical Infectious Diseases. 2009;48(7):879–87. doi: 10.1086/597260 [DOI] [PubMed] [Google Scholar]
- 51.Schlüter D, Oprisiu SB, Chahoud S, Weiner D, Wiestler OD, Hof H, et al. Systemic immunization induces protective CD4+ and CD8+ T cell‐mediated immune responses in murine Listeria monocytogenes meningoencephalitis. European Journal of Immunology. 1995;25(8):2384–91. doi: 10.1002/eji.1830250839 [DOI] [PubMed] [Google Scholar]
- 52.Sasseville V, Smith M, Mackav C, Ringler D, Lackner A. Chemokines expression in SIV-lnduced AIDS Encephalitis. FASEB Journal. 1996;10(6). [PMC free article] [PubMed] [Google Scholar]
- 53.Rosler A, Pohl M, Braune HJ, Oertel WH, Gemsa D, Sprenger H. Time course of chemokines in the cerebrospinal fluid and serum during herpes simplex type 1 encephalitis. J Neurol Sci. 1998;157(1):82–9. doi: 10.1016/s0022-510x(98)00061-6 [DOI] [PubMed] [Google Scholar]
- 54.Ravi V, Parida S, Desai A, Chandramuki A, Gourie-Devi M, Grau GE. Correlation of tumor necrosis factor levels in the serum and cerebrospinal fluid with clinical outcome in Japanese encephalitis patients. J Med Virol. 1997;51(2):132–6. [PubMed] [Google Scholar]
- 55.Ramaswamy V, Walsh JG, Sinclair DB, Johnson E, Tang-Wai R, Wheatley BM, et al. Inflammasome induction in Rasmussen’s encephalitis: cortical and associated white matter pathogenesis. J Neuroinflamm. 2013;10:10. doi: 10.1186/1742-2094-10-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pujhari SK, Prabhakar S, Ratho R, Mishra B, Modi M, Sharma S, et al. Th1 immune response takeover among patients with severe Japanese encephalitis infection. J Neuroimmunol. 2013;263(1–2):133–8. doi: 10.1016/j.jneuroim.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 57.Pohl-Koppe A, Burchett SK, Thiele EA, Hafler DA. Myelin basic protein reactive Th2 T cells are found in acute disseminated encephalomyelitis. J Neuroimmunol. 1998;91(1–2):19–27. doi: 10.1016/s0165-5728(98)00125-8 [DOI] [PubMed] [Google Scholar]
- 58.Pilz G, Wipfler P, Otto F, Hitzl W, Afazel S, Haschke-Becher E, et al. Cerebrospinal fluid CXLC13 indicates disease course in neuroinfection: an observational study. J Neuroinflamm. 2019;16:5. doi: 10.1186/s12974-019-1405-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petito CK, Roberts B, Cantando JD, Rabinstein A, Duncan R. Hippocampal injury and alterations in neuronal chemokine co-receptor expression in patients with AIDS. J Neuropathol Exp Neurol. 2001;60(4):377–85. doi: 10.1093/jnen/60.4.377 [DOI] [PubMed] [Google Scholar]
- 60.Park SY, Kwon JS, Kim JY, Kim SM, Jang YR, Kim MC, et al. Severe fever with thrombocytopenia syndrome-associated encephalopathy/encephalitis. Clin Microbiol Infect. 2018;24(4):4. doi: 10.1016/j.cmi.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 61.Pandey AK, Ansari Z, Kushwaha KP, Gore M. Determination of the cytokines and chemokines level in the serum and csf of JE and non-JE viral encephalitis cases. Cytokine. 2012;59(3):520–. [Google Scholar]
- 62.Owens GC, Huynh MN, Chang JW, McArthur DL, Hickey MJ, Vinters HV, et al. Differential expression of interferon-gamma and chemokine genes distinguishes Rasmussen encephalitis from cortical dysplasia and provides evidence for an early Th1 immune response. J Neuroinflamm. 2013;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nuovo GJ, DeFaria DL, Chanona-Vilchi JG, Zhang Y. Molecular detection of rabies encephalitis and correlation with cytokine expression. Modern Pathology. 2005;18(1):62–7. doi: 10.1038/modpathol.3800274 [DOI] [PubMed] [Google Scholar]
- 64.Mori D, Khanam W, Sheikh RA, Bin Tabib SMS, Ikebe E, Hossain MM, et al. Increased serum vascular endothelial growth factor is associated with acute viral encephalitis in Bangladeshi children. Sci Rep. 2017;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moniuszko A, Pancewicz S, Czupryna P, Grygorczuk S, Swierzbinska R, Kondrusik M, et al. ssICAM-1, IL-21 and IL-23 in patients with tick borne encephalitis and neuroborreliosis. Cytokine. 2012;60(2):468–72. doi: 10.1016/j.cyto.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miyata R, Tanuma N, Hayashi M, Imamura T, Takanashi J, Nagata R, et al. Oxidative stress in patients with clinically mild encephalitis/encephalopathy with a reversible splenial lesion (MERS). Brain Dev. 2012;34(2):124–7. doi: 10.1016/j.braindev.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 67.Mishra MK, Kumawat KL, Basu A. Japanese encephalitis virus differentially modulates the induction of multiple pro-inflammatory mediators in human astrocytoma and astroglioma cell-lines. Cell Biol Int. 2008;32(12):1506–13. doi: 10.1016/j.cellbi.2008.08.020 [DOI] [PubMed] [Google Scholar]
- 68.Mirones I, de Prada I, Gómez AM, Luque A, Martín R, Pérez-Jiménez M, et al. A role for the CXCR3/CXCL10 axis in Rasmussen encephalitis. Pediatr Neurol. 2013;49(6):451–7.e1. doi: 10.1016/j.pediatrneurol.2013.07.019 [DOI] [PubMed] [Google Scholar]
- 69.Mine J, Takahashi Y, Mogami Y, Ikeda H, Kubota Y, Imai K. Characteristics of epilepsy and immunological markers in epileptic patients after influenza-associated encephalopathy. Neurol Asia. 2013;18(1):35–45. [Google Scholar]
- 70.Mickiene A, Pakalniene J, Nordgren J, Carlsson B, Hagbom M, Svensson L, et al. Polymorphisms in Chemokine Receptor 5 and Toll-Like Receptor 3 Genes Are Risk Factors for Clinical Tick-Borne Encephalitis in the Lithuanian Population. PLoS One. 2014;9(9):10. doi: 10.1371/journal.pone.0106798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Michael BD, Griffiths MJ, Granerod J, Brown D, Keir G, Wnek G, et al. The Interleukin-1 Balance During Encephalitis Is Associated With Clinical Severity, Blood-Brain Barrier Permeability, Neuroimaging Changes, and Disease Outcome. J Infect Dis. 2016;213(10):1651–60. doi: 10.1093/infdis/jiv771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matsuzono Y, Narita M, Akutsu Y, Togashi T. Interleukin-6 in cerebrospinal fluid of patients with central nervous system infections. Acta Paediatrica, International Journal of Paediatrics. 1995;84(8):879–83. doi: 10.1111/j.1651-2227.1995.tb13784.x [DOI] [PubMed] [Google Scholar]
- 73.Marques CP, Hu S, Sheng W, Cheeran MC, Cox D, Lokensgard JR. Interleukin-10 attenuates production of HSV-induced inflammatory mediators by human microglia. Glia. 2004;47(4):358–66. doi: 10.1002/glia.20045 [DOI] [PubMed] [Google Scholar]
- 74.Luo Y, Yan W, Zhou Z, Liu B, Wang Z, Chen J, et al. Elevated levels of NLRP3 in cerebrospinal fluid of patients with autoimmune GFAP astrocytopathy. Frontiers in Neurology. 2019;10(OCT). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu SC, Lee PI, Lee CY, Wang JD, Chiang BL, Chou MC. Different cytokine levels in enterovirus meningitis and encephalitis. Infectious Diseases in Clinical Practice. 2005;13(5):241–6. [Google Scholar]
- 76.Lind L, Studahl M, Persson Berg L, Eriksson K. CXCL11 production in cerebrospinal fluid distinguishes herpes simplex meningitis from herpes simplex encephalitis. J Neuroinflamm. 2017;14(1). doi: 10.1186/s12974-017-0907-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li JY, Gu Y, An HW, Zhou ZY, Zheng D, Wang ZH, et al. Cerebrospinal fluid light and heavy neurofilament level increased in anti-N-methyl-d-aspartate receptor encephalitis. Brain Behav. 2019;9(8):8. doi: 10.1002/brb3.1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumar P, Sulochana P, Nirmala G, Chandrashekar R, Haridattatreya M, Satchidanandam V. Impaired T helper 1 function of nonstructural protein 3-specific T cells in Japanese patients with encephalitis with neurological sequelae. J Infect Dis. 2004;189(5):880–91. doi: 10.1086/381768 [DOI] [PubMed] [Google Scholar]
- 79.Kubota H, Tanabe Y, Komiya T, Hirai K, Takanashi J, Kohno Y. Q fever encephalitis with cytokine profiles in serum and cerebrospinal fluid. Pediatr Infect Dis J. 2001;20(3):318–9. doi: 10.1097/00006454-200103000-00022 [DOI] [PubMed] [Google Scholar]
- 80.Kortvelyessy P, Goihl A, Guttek K, Schraven B, Pruss H, Reinhold D. Serum and CSF cytokine levels mirror different neuroimmunological mechanisms in patients with LGI1 and Caspr2 encephalitis. Cytokine. 2020;135:8. doi: 10.1016/j.cyto.2020.155226 [DOI] [PubMed] [Google Scholar]
- 81.Kawahara Y, Morimoto A, Oh Y, Furukawa R, Wakabayashi K, Monden Y, et al. Serum and cerebrospinal fluid cytokines in children with acute encephalopathy. Brain Dev. 2020;42(2):185–91. doi: 10.1016/j.braindev.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 82.Kawabe S, Ito Y, Ohta R, Sofue A, Gotoh K, Morishima T, et al. Comparison of the Levels of Human Herpesvirus 6 (HHV-6) DNA and Cytokines in the Cerebrospinal Fluid and Serum of Children With HHV-6 Encephalopathy. J Med Virol. 2010;82(8):1410–5. doi: 10.1002/jmv.21808 [DOI] [PubMed] [Google Scholar]
- 83.Kang XP, Li YC, Wei JJ, Zhang Y, Bian C, Wang K, et al. Elevation of Matrix Metalloproteinase-9 Level in Cerebrospinal Fluid of Tick-Borne Encephalitis Patients Is Associated with IgG Extravassation and Disease Severity. PLoS One. 2013;8(11):5. doi: 10.1371/journal.pone.0077427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson MD, Kim P, Tourtellotte W, Federspiel CF. Transforming growth factor β and monocyte chemotactic protein-1 are elevated in cerebrospinal fluid of immunocompromised patients with HIV-1 infection. Journal of Neuro-AIDS. 2004;2(4):33–43. [DOI] [PubMed] [Google Scholar]
- 85.Hua Y, Wang Y, Gong W. Inflammatory cytokine profiles of serum and cerebrospinal fluid in Chinese children with hand, foot and mouth disease. Int J Clin Exp Pathol. 2017;10(11):11022–9. [PMC free article] [PubMed] [Google Scholar]
- 86.Hu MH, Huang GS, Wu CT, Lin JJ, Hsia SH, Wang HS, et al. Analysis of plasma multiplex cytokines for children with febrile seizures and severe acute encephalitis. Journal of Child Neurology. 2014;29(2):182–6. doi: 10.1177/0883073813488829 [DOI] [PubMed] [Google Scholar]
- 87.Hansen N, Timäus C. New aspects of biological markers in autoimmune encephalitis. Zeitschrift fur Epileptologie. 2020. [Google Scholar]
- 88.Grygorczuk S, Osada J, Parczewski M, Moniuszko A, Swierzbinska R, Kondrusik M, et al. The expression of the chemokine receptor CCR5 in tick-borne encephalitis. J Neuroinflamm. 2016;13:17. doi: 10.1186/s12974-016-0511-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Griffiths MJ, Ooi MH, Wong SC, Mohan A, Podin Y, Perera D, et al. In Enterovirus 71 Encephalitis With Cardio-Respiratory Compromise, Elevated Interleukin 1 beta, Interleukin 1 Receptor Antagonist, and Granulocyte Colony-Stimulating Factor Levels Are Markers of Poor Prognosis. J Infect Dis. 2012;206(6):881–92. [DOI] [PubMed] [Google Scholar]
- 90.Fowler A, Ygberg S, Bogdanovic G, Wickstrom R. Biomarkers in Cerebrospinal Fluid of Children With Tick-borne Encephalitis: Association With Long-term Outcome. Pediatr Infect Dis J. 2016;35(9):961–6. doi: 10.1097/INF.0000000000001210 [DOI] [PubMed] [Google Scholar]
- 91.Cui N, Liu R, Lu QB, Wang LY, Qin SL, Yang ZD, et al. Severe fever with thrombocytopenia syndrome bunyavirus-related human encephalitis. Journal of Infection. 2014. doi: 10.1016/j.jinf.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 92.Chowdhury P, Khan SA. Significance of CCL2, CCL5 and CCR2 polymorphisms for adverse prognosis of Japanese encephalitis from an endemic population of India. Sci Rep. 2017;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen Z, Zhong D, Li G. The role of microglia in viral encephalitis: a review. Journal of neuroinflammation. 2019;16(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Canessa A, Del Bono V, Miletich F, Pistoia V. Serum cytokines in toxoplasmosis: increased levels of interferon-gamma in immunocompetent patients with lymphadenopathy but not in AIDS patients with encephalitis. J Infect Dis. 1992;165(6):1168–70. doi: 10.1093/infdis/165.6.1168 [DOI] [PubMed] [Google Scholar]
- 95.Bogovic P, Lusa L, Korva M, Lotric-Furlan S, Resman-Rus K, Pavletic M, et al. Inflammatory Immune Responses in Patients with Tick-Borne Encephalitis: Dynamics and Association with the Outcome of the Disease. Microorganisms. 2019;7(11):16. doi: 10.3390/microorganisms7110514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aberle JH, Schwaiger J, Aberle SW, Stiasny K, Scheinost O, Kundi M, et al. Human CD4(+) T Helper Cell Responses after Tick-Borne Encephalitis Vaccination and Infection. PLoS One. 2015;10(10):15. doi: 10.1371/journal.pone.0140545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bodro M, Compta Y, Llanso L, Esteller D, Doncel-Moriano A, Mesa A, et al. Increased CSF levels of IL-1 beta, IL-6, and ACE in SARS-CoV-2-associated encephalitis. Neurol-Neuroimmunol Neuroinflammation. 2020;7(5):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fujimori J, Takahashi T, Kaneko K, Atobe Y, Nakashima I. Anti-NMDAR encephalitis may develop concurrently with anti-MOG antibody-associated bilateral medial frontal cerebral cortical encephalitis and relapse with elevated CSF IL-6 and CXCL13. Multiple Sclerosis and Related Disorders. 2021;47. doi: 10.1016/j.msard.2020.102611 [DOI] [PubMed] [Google Scholar]
- 99.Leis AA, Grill MF, Goodman BP, Sadiq SB, Sinclair DJ, Vig PJS, et al. Tumor Necrosis Factor-Alpha Signaling May Contribute to Chronic West Nile Virus Post-infectious Proinflammatory State. Front Med. 2020;7:6. doi: 10.3389/fmed.2020.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Winter PM, Nguyen MD, Ha TL, Kneen R, Wills B, Le TT, et al. Proinflammatory cytokines and chemokines in humans with Japanese encephalitis. J Infect Dis. 2004;190(9):1618–26. doi: 10.1086/423328 [DOI] [PubMed] [Google Scholar]
- 101.Takano K, Ogata M, Satou T, Miyazaki Y, Otsuka E, Saito N, et al. Correlations of cytokine levels in cerebrospinal fluid and peripheral blood with outcome of HHV-6B encephalitis after hematopoietic stem cell transplantation. Transplant Infectious Disease. 2019;21(6). doi: 10.1111/tid.13172 [DOI] [PubMed] [Google Scholar]
- 102.Li YY, Li HP, Wen B, Zhang J, Li SC, Xie Y, et al. Cytokine expression profile in hospitalized children with herpes simplex virus-1 infection. Future Virol. 2017;12(9):485–90. [Google Scholar]
- 103.Atrasheuskaya AV, Fredeking IM, Ignatyev GM. Changes in immune parameters and their correction in human cases of tick-borne encephalitis. Clin Exp Immunol. 2003;131(1):148–54. doi: 10.1046/j.1365-2249.2003.02050.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zajkowska J, Grygorczuk S, Pryszmont JM, Kondrusik M, Pancewicz S, Świerzbińska R, et al. Concentration of interleukin 6 and 10 in tick-borne and purulend encephalomeningitis. Polski Merkuriusz Lekarski. 2006;21(121):29–34. [PubMed] [Google Scholar]
- 105.Timofeev AV, Kondratyeva YY, Orlovsky VG, Kurzhukov GP, Loktev VB, Karganova GG. Correlations between tick-borne encephalitis severity and concentration of interleukines 2 and 6 in the serum of patients with this disease. Ter Arkhiv. 2002;74(11):22–3. [PubMed] [Google Scholar]
- 106.Riazantseva NV, Novitskiǐ VV, Zima AP, Zhukova NG, Pirogova NP, Naslednikova IO, et al. Dysregulation of cytokine production by mononuclear cells of peripheral blood in persistent infection with a virus of tick-borne encephalitis. Zhurnal nevrologii i psikhiatrii imeni SS Korsakova / Ministerstvo zdravookhraneniia i meditsinskoǐ promyshlennosti Rossiǐskoǐ Federatsii, Vserossiǐskoe obshchestvo nevrologov [i] Vserossiǐskoe obshchestvo psikhiatrov. 2006;106(12):57–62. [PubMed] [Google Scholar]
- 107.Poponnikova TV, Bedareva TY, Vakhrameeva TN, Galieva GY. The cytokine profile in the acute period of tick-borne neuroinfections in children. Z Nevrol Psikhiatrii Im S S Korsakova. 2010;110(5):9–12. [PubMed] [Google Scholar]
- 108.Pietruczuk M, Pietruczuk A, Pancewicz S, Zajkowska J, Swierzbińska R, Hermanowska-Szpakowicz T. Intercellular adhesion molecules sICAM-1, sICAM-2, sICAM-3 and IFNgamma in neuroborreliosis and tick-borne encephalitis. Przegląd epidemiologiczny. 2006;60 Suppl 1:109–17. [PubMed] [Google Scholar]
- 109.Mizuguchi M. [Overview of acute encephalitis and encephalopathy]. Nippon rinsho Japanese journal of clinical medicine. 2011;69(3):391–8. [PubMed] [Google Scholar]
- 110.Michałowska-Wender G, Losy J, Kondrusik M, Zajkowska J, Pancewicz S, Grygorczuk S, et al. Evaluation of soluble platelet cell adhesion molecule sPECAM-1 and chemokine MCP-1 (CCL2) concentration in CSF of patients with tick-borne encephalitis. Polski Merkuriusz Lekarski. 2006;20(115):46–8. [PubMed] [Google Scholar]
- 111.Lin YX, Ye ZX, Kang DZ, Lin K, Wang F. Expression and clinical significance of tumor necrosis factor-alpha and its receptors in non-specific chronic encephalitis and non-encephalitis relative intractable epilepsy. National Medical Journal of China. 2013;93(39):3122–4. [PubMed] [Google Scholar]
- 112.Kondrusik M, Pancewicz S, Zajkowska J, Hermanowska-Szpakowicz T. Tumor necrosis factor alpha and interleukin 1-beta in serum of patients with tick-borne encephalitis. Polski merkuriusz lekarski: organ Polskiego Towarzystwa Lekarskiego. 2001;11(61):26–8. [PubMed] [Google Scholar]
- 113.Kondrusik M, Hermanowska-Szpakowicz T, Jaroszewicz E. Concentrations of tumor necrosis factor alpha and interleukin-1 beta in cerebrospinal fluid in the course of tick-borne encephalitis. Polski merkuriusz lekarski: organ Polskiego Towarzystwa Lekarskiego. 1998;4(21):126–9. [PubMed] [Google Scholar]
- 114.Khaled M, Youssof AMA, Mourad HS. Clinical and laboratory predictors of outcome in patients with herpes simplex encephalitis. Egyptian Journal of Neurology, Psychiatry and Neurosurgery. 2014;51(2):173–80. [Google Scholar]
- 115.Kepa L, Adamek B. Evaluation of tumor necrosis factor and C-reactive protein level determination in cerebrospinal fluid of meningitis and encephalitis. Polski merkuriusz lekarski: organ Polskiego Towarzystwa Lekarskiego. 1997;2(12):359–62. [PubMed] [Google Scholar]
- 116.Ichiyama T, Nishikawa M, Hayashi T, Furukawa S. Proinflammatory cytokine levels in cerebrospinal fluid from children with acute encephalitis. No To Hattatsu. 1997;29(6):466–70. [PubMed] [Google Scholar]
- 117.Grygorczuk S, Zajkowska J, Świerzbińska R, Pancewicz S, Kondrusik M, Hermanowska-Szpakowicz T. Concentration of the beta-chemokine CCL5 (RANTES) in cerebrospinal fluid in patients with thick-borne encephalitis. Neurologia i Neurochirurgia Polska. 2006;40(2):106–11. [PubMed] [Google Scholar]
- 118.Grygorczuk S, Zajkowska J, Swierzbińska R, Pancewicz S, Kondrusik M, Hermanowska-Szpakowicz T. Elevated concentration of the chemokine CCL3 (MIP-1alpha) in cerebrospinal fluid and serum of patients with tick borne encephalitis. Advances in medical sciences. 2006;51:340–4. [PubMed] [Google Scholar]
- 119.Brown DA, Jiang J, Ramanathan S, Silvestrini R, Bleasel A, Wienholt L, et al. Potential surrogate markers of central nervous system inflammation in cerebrospinal fluid in the diagnosis of autoimmune encephalitis. Neurology Psychiatry and Brain Research. 2018;29:6–7. [Google Scholar]
- 120.Aurelius E. Herpes simplex encephalitis. Early diagnosis and immune activation in the acute stage and during long-term follow-up. Scandinavian Journal of Infectious Diseases, Supplement. 1993(89):3–62. [PubMed] [Google Scholar]
- 121.Grygorczuk S, Osada J, Toczylowski K, Sulik A, Czupryna P, Moniuszko-Malinowska A, et al. The lymphocyte populations and their migration into the central nervous system in tick-borne encephalitis. Ticks Tick-Borne Dis. 2020;11(5):14. doi: 10.1016/j.ttbdis.2020.101467 [DOI] [PubMed] [Google Scholar]
- 122.Levraut M, Bourg V, Capet N, Delourme A, Honnorat J, Thomas P, et al. Cerebrospinal Fluid IL-17A Could Predict Acute Disease Severity in Non-NMDA-Receptor Autoimmune Encephalitis. Front Immunol. 2021;12:8. doi: 10.3389/fimmu.2021.673021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Westman G, Aurelius E, Ahlm C, Blennow K, Eriksson K, Lind L, et al. Cerebrospinal fluid biomarkers of brain injury, inflammation and synaptic autoimmunity predict long-term neurocognitive outcome in herpes simplex encephalitis. Clin Microbiol Infect. 2021;27(8):1131–6. doi: 10.1016/j.cmi.2020.09.031 [DOI] [PubMed] [Google Scholar]
- 124.Zhang D, Zheng N, Liu X. The role and mechanism of NF-κB in viral encephalitis of children. Experimental and Therapeutic Medicine. 2017;13(6):3489–93. doi: 10.3892/etm.2017.4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ygberg S, Fowler A, Wickstrom R. Cytokine and Chemokine Expression in CSF May Differentiate Viral and Autoimmune NMDAR Encephalitis in Children. Journal of Child Neurology. 2016;31(13):1450–6. doi: 10.1177/0883073816653780 [DOI] [PubMed] [Google Scholar]
- 126.Ygberg S, Fowler A, Bogdanovic G, Wickstrom R. The Cerebrospinal Fluid Interleukin-6/Interleukin-10 Ratio Differentiates Pediatric Tick-borne Infections. Pediatr Infect Dis J. 2020;39(3):239–43. doi: 10.1097/INF.0000000000002552 [DOI] [PubMed] [Google Scholar]
- 127.Wang SM, Lei HY, Yu CK, Wang JR, Su IJ, Liu CC. Acute chemokine response in the blood and cerebrospinal fluid of children with enterovirus 71-associated Brainstem encephalitis. J Infect Dis. 2008;198(7):1002–6. doi: 10.1086/591462 [DOI] [PubMed] [Google Scholar]
- 128.Wang SM, Lei HY, Su LY, Wu JM, Yu CK, Wang JR, et al. Cerebrospinal fluid cytokines in enterovirus 71 brain stem encephalitis and echovirus meningitis infections of varying severity. Clin Microbiol Infect. 2007;13(7):677–82. doi: 10.1111/j.1469-0691.2007.01729.x [DOI] [PubMed] [Google Scholar]
- 129.Ulusoy C, Tüzün E, Kürtüncü M, Türkoğlu R, Akman-Demir G, Eraksoy M. Comparison of the cytokine profiles of patients with neuronal-antibody-associated central nervous system disorders. International Journal of Neuroscience. 2012;122(6):284–9. doi: 10.3109/00207454.2011.648762 [DOI] [PubMed] [Google Scholar]
- 130.Tripathy A, Balaji S, Rao N, Thakare J, Mishra A, Arankalle V. Cytokine levels in Chandipura virus associated encephalopathy in children. Scand J Infect Dis. 2005;37(8):590–3. doi: 10.1080/00365540510044067 [DOI] [PubMed] [Google Scholar]
- 131.Singh SK, Kulshreshtha D, Singh AK, Maurya PK, Thacker AK. Acute Encephalitis Syndrome in Adults and Its Correlation with Cytokine Levels in the Serum and Cerebrospinal Fluid. Jpn J Infect Dis. 2017;70(4):374–7. doi: 10.7883/yoken.JJID.2016.063 [DOI] [PubMed] [Google Scholar]
- 132.Shang W, Qian S, Fang L, Han Y, Zheng C. Association study of inflammatory cytokine and chemokine expression in hand foot and mouth disease. Oncotarget. 2017;8(45):79425–32. doi: 10.18632/oncotarget.18341 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 133.Salgado DM, Vega R, Rodriguez JA, Nino A, Rodriguez R, Ortiz A, et al. Clinical, laboratory and immune aspects of Zika virus-associated encephalitis in children. Int J Infect Dis. 2020;90:104–10. doi: 10.1016/j.ijid.2019.10.030 [DOI] [PubMed] [Google Scholar]
- 134.Peng Y, Zheng D, Zhang XM, Pan SY, Ji T, Zhang J, et al. Cell-Free Mitochondrial DNA in the CSF: A Potential Prognostic Biomarker of Anti-NMDAR Encephalitis. Front Immunol. 2019;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Peng Y, Liu B, Pei S, Zheng D, Wang Z, Ji T, et al. Higher CSF Levels of NLRP3 Inflammasome Is Associated with Poor Prognosis of Anti-N-methyl-D-Aspartate Receptor Encephalitis. Front Immunol. 2019;10(MAY). doi: 10.3389/fimmu.2019.00905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Palus M, Formanova P, Salat J, Zampachova E, Elsterova J, Ruzek D. Analysis of Serum Levels of Cytokines, Chemokines, Growth Factors, and Monoamine Neurotransmitters in Patients With Tick-borne Encephalitis: Identification of Novel Inflammatory Markers With Implications for Pathogenesis. J Med Virol. 2015;87(5):885–92. doi: 10.1002/jmv.24140 [DOI] [PubMed] [Google Scholar]
- 137.Narita M, Tanaka H, Togashi T, Abe S. Cytokines involved in CNS manifestations caused by Mycoplasma pneumoniae. Pediatr Neurol. 2005;33(2):105–9. doi: 10.1016/j.pediatrneurol.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 138.Michael BD, Griffiths MJ, Granerod J, Brown D, Davies NW, Borrow R, et al. Characteristic Cytokine and Chemokine Profiles in Encephalitis of Infectious, Immune-Mediated, and Unknown Aetiology. PLoS One. 2016;11(1):e0146288. doi: 10.1371/journal.pone.0146288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Matsui M, Tanaka K, Nagumo F, Kuroda Y. Central nervous system immunity associated with clinical outcome in acute encephalitis. J Neurol Sci. 2004;227(1):139–47. doi: 10.1016/j.jns.2004.09.022 [DOI] [PubMed] [Google Scholar]
- 140.Liu J, Li S, Cai C, Xu Y, Jiang Y, Chen Z. Cerebrospinal fluid chemokine patterns in children with enterovirus 71-related encephalitis. Sci Rep. 2018;8(1):1658. doi: 10.1038/s41598-018-19988-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu B, Ai P, Zheng D, Jiang Y, Liu X, Pan S, et al. Cerebrospinal fluid pentraxin 3 and CD40 ligand in anti-N-menthyl-D-aspartate receptor encephalitis. J Neuroimmunol. 2018;315:40–4. doi: 10.1016/j.jneuroim.2017.11.016 [DOI] [PubMed] [Google Scholar]
- 142.Lin TY, Chang LY, Huang YC, Hsu KH, Chiu CH, Yang KD. Different proinflammatory reactions in fatal and non-fatal enterovirus 71 infections: implications for early recognition and therapy. Acta Paediatr. 2002;91(6):632–5. doi: 10.1080/080352502760069016 [DOI] [PubMed] [Google Scholar]
- 143.Liba Z, Kayserova J, Elisak M, Marusic P, Nohejlova H, Hanzalova J, et al. Anti-N-methyl-D-aspartate receptor encephalitis: the clinical course in light of the chemokine and cytokine levels in cerebrospinal fluid. J Neuroinflamm. 2016;13:8. doi: 10.1186/s12974-016-0507-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li J, Zhang N, Hao XW, Yang ZZ, Yan XJ, Zhang YL. Predictive value of procalcitonin, tumor necrosis factor-alpha and neuron-specific enolase levels in cerebrospinal fluid and serum for viral encephalitis severity and prognosis. Int J Clin Exp Med. 2020;13(3):1897–904. [Google Scholar]
- 145.Li H, Li Y, Wen B, Zhang J, Wang C, Song Z, et al. Dengue virus and Japanese encephalitis virus infection of the central nervous system share similar profiles of cytokine accumulation in cerebrospinal fluid. Central European Journal of Immunology. 2017;42(2):218–22. doi: 10.5114/ceji.2017.69366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Leypoldt F, Hoftberger R, Titulaer MJ, Armangue T, Gresa-Arribas N, Jahn H, et al. Investigations on CXCL13 in Anti-N-Methyl-D-Aspartate Receptor Encephalitis A Potential Biomarker of Treatment Response. JAMA Neurol. 2015;72(2):180–6. doi: 10.1001/jamaneurol.2014.2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lepej SZ, Misic-Majerus L, Jeren T, Rode OD, Remenar A, Sporec V, et al. Chemokines CXCL10 and CXCL11 in the cerebrospinal fluid of patients with tick-borne encephalitis. Acta Neurol Scand. 2007;115(2):109–14. doi: 10.1111/j.1600-0404.2006.00726.x [DOI] [PubMed] [Google Scholar]
- 148.Lebon P, Boutin B, Dulac O, Ponsot G, Arthuis M. Interferon gamma in acute and subacute encephalitis. Br Med J (Clin Res Ed). 1988;296(6614):9–11. doi: 10.1136/bmj.296.6614.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Leake JA, Albani S, Kao AS, Senac MO, Billman GF, Nespeca MP, et al. Acute disseminated encephalomyelitis in childhood: epidemiologic, clinical and laboratory features. Pediatr Infect Dis J. 2004;23(8):756–64. doi: 10.1097/01.inf.0000133048.75452.dd [DOI] [PubMed] [Google Scholar]
- 150.Kothur K, Wienholt L, Mohammad SS, Tantsis EM, Pillai S, Britton PN, et al. Utility of CSF Cytokine/Chemokines as Markers of Active Intrathecal Inflammation: Comparison of Demyelinating, Anti-NMDAR and Enteroviral Encephalitis. PLoS One. 2016;11(8):19. doi: 10.1371/journal.pone.0161656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kothur K, Gill D, Wong M, Mohammad SS, Bandodkar S, Arbunckle S, et al. Cerebrospinal fluid cyto‐/chemokine profile during acute herpes simplex virus induced anti‐N‐methyl‐d‐aspartate receptor encephalitis and in chronic neurological sequelae. Developmental Medicine & Child Neurology. 2017;59(8):806–14. [DOI] [PubMed] [Google Scholar]
- 152.Koper OM, Kaminska J, Grygorczuk S, Zajkowska J, Kemona H. CXCL9 concentrations in cerebrospinal fluid and serum of patients with tick-borne encephalitis. Arch Med Sci. 2018;14(2):313–20. doi: 10.5114/aoms.2016.58667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kawamura Y, Sugata K, Ihira M, Mihara T, Mutoh T, Asano Y, et al. Different characteristics of human herpesvirus 6 encephalitis between primary infection and viral reactivation. Journal of Clinical Virology. 2011;51(1):12–9. doi: 10.1016/j.jcv.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 154.Kamei S, Taira N, Ishihara M, Sekizawa T, Morita A, Miki K, et al. Prognostic value of cerebrospinal fluid cytokine changes in herpes simplex virus encephalitis. Cytokine. 2009;46(2):187–93. doi: 10.1016/j.cyto.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 155.Kalita J, Srivastava R, Mishra MK, Basu A, Misra UK. Cytokines and chemokines in viral encephalitis: A clinicoradiological correlation. Neurosci Lett. 2010;473(1):48–51. doi: 10.1016/j.neulet.2010.02.017 [DOI] [PubMed] [Google Scholar]
- 156.Jiang XY, Lei S, Zhang L, Liu X, Lin MT, Blumcke I, et al. Co-expression of NMDA-receptor subunits NR1, NR2A, and NR2B in dysplastic neurons of teratomas in patients with paraneoplastic NMDA-receptor-encephalitis: a retrospective clinico-pathology study of 159 patients. Acta Neuropathol Commun. 2020;8(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jiang JX, Fewings N, Dervish S, Fois AF, Duma SR, Silsby M, et al. Novel Surrogate Markers of CNS Inflammation in CSF in the Diagnosis of Autoimmune Encephalitis. Front Neurol. 2019;10:1390. doi: 10.3389/fneur.2019.01390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ishizu T, Minohara M, Ichiyama T, Kira R, Tanaka M, Osoegawa M, et al. CSF cytokine and chemokine profiles in acute disseminated encephalomyelitis. J Neuroimmunol. 2006;175(1–2):52–8. doi: 10.1016/j.jneuroim.2006.03.020 [DOI] [PubMed] [Google Scholar]
- 159.Ichiyama T, Shoji H, Takahashi Y, Matsushige T, Kajimoto M, Inuzuka T, et al. Cerebrospinal fluid levels of cytokines in non-herpetic acute limbic encephalitis: Comparison with herpes simplex encephalitis. Cytokine. 2008;44(1):149–53. doi: 10.1016/j.cyto.2008.07.002 [DOI] [PubMed] [Google Scholar]
- 160.Ichiyama T, Shoji H, Kato M, Sawaishi Y, Ozawa H, Matsubara T, et al. Cerebrospinal fluid levels of cytokines and soluble tumour necrosis factor receptor in acute disseminated encephalomyelitis. Eur J Pediatr. 2002;161(3):133–7. doi: 10.1007/s00431-001-0888-2 [DOI] [PubMed] [Google Scholar]
- 161.Ichiyama T, Nishikawa M, Yoshitomi T, Hayashi T, Furukawa S. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 in cerebrospinal fluid from children with prolonged febrile seizures—Comparison with acute encephalitis/encephalopathy. Neurology. 1998;50(2):407–11. [DOI] [PubMed] [Google Scholar]
- 162.Holub M, Beran O, Lacinová Z, Cinek O, Chalupa P. Interferon-gamma and cortisol levels in cerebrospinal fluid and its relationship to the etiology of aseptic meningoencephalitis. Prague medical report. 2006;107(3):343–53. [PubMed] [Google Scholar]
- 163.Günther G, Haglund M, Lindquist L, Forsgren M, Andersson J, Andersson B, et al. Tick-borne encephalitis is associated with low levels of interleukin-10 in cerebrospinal fluid.?. 2011;1. doi: 10.3402/iee.v1i0.6029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Grygorczuk S, Swierzbinska R, Kondrusik M, Dunaj J, Czupryna P, Moniuszko A, et al. The intrathecal expression and pathogenetic role of Th17 cytokines and CXCR2-binding chemokines in tick-borne encephalitis. J Neuroinflamm. 2018;15:20. doi: 10.1186/s12974-018-1138-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Grygorczuk S, Parczewski M, Swierzbinska R, Czupryna P, Moniuszko A, Dunaj J, et al. The increased concentration of macrophage migration inhibitory factor in serum and cerebrospinal fluid of patients with tick-borne encephalitis. J Neuroinflamm. 2017;14:17. doi: 10.1186/s12974-017-0898-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Grygorczuk S, Parczewski M, Moniuszko A, Swierzbinska R, Kondrusik M, Zajkowska J, et al. Increased concentration of interferon lambda-3, interferon beta and interleukin-10 in the cerebrospinal fluid of patients with tick-borne encephalitis. Cytokine. 2015;71(2):125–31. doi: 10.1016/j.cyto.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 167.Grygorczuk S, Czupryna P, Pancewicz S, Swierzbinska R, Kondrusik M, Dunaj J, et al. Intrathecal expression of IL-5 and humoral response in patients with tick-borne encephalitis. Ticks Tick-Borne Dis. 2018;9(4):896–911. doi: 10.1016/j.ttbdis.2018.03.012 [DOI] [PubMed] [Google Scholar]
- 168.Fominykh V, Brylev L, Gaskin V, Luzin R, Yakovlev A, Komoltsev I, et al. Neuronal damage and neuroinflammation markers in patients with autoimmune encephalitis and multiple sclerosis. Metab Brain Dis. 2019;34(5):1473–85. doi: 10.1007/s11011-019-00452-x [DOI] [PubMed] [Google Scholar]
- 169.Dale RC, Morovat A. Interleukin-6 and oligoclonal IgG synthesis in children with acute disseminated encephalomyelitis. Neuropediatrics. 2003;34(3):141–5. doi: 10.1055/s-2003-41281 [DOI] [PubMed] [Google Scholar]
- 170.Courtioux B, Pervieux L, Vatunga G, Marin B, Josenando T, Jauberteau-Marchan MO, et al. Increased CXCL-13 levels in human African trypanosomiasis meningo-encephalitis. Trop Med Int Health. 2009;14(5):529–34. doi: 10.1111/j.1365-3156.2009.02263.x [DOI] [PubMed] [Google Scholar]
- 171.Chowdhury P, Khan SA. Differential Expression Levels of Inflammatory Chemokines and TLRs in Patients Suffering from Mild and Severe Japanese Encephalitis. Viral Immunol. 2019;32(1):68–74. doi: 10.1089/vim.2018.0103 [DOI] [PubMed] [Google Scholar]
- 172.Byun J-I, Lee S-T, Moon J, Jung K-H, Sunwoo J-S, Lim J-A, et al. Distinct intrathecal interleukin-17/interleukin-6 activation in anti-N-methyl-d-aspartate receptor encephalitis. Journal of neuroimmunology. 2016;297:141–7. doi: 10.1016/j.jneuroim.2016.05.023 [DOI] [PubMed] [Google Scholar]
- 173.Bociaga-Jasik M, Ciesla A, Kalinowska-Nowak A, Skwara P, Garlicki A, Mach T. Role of IL-6 and neopterin in the pathogenesis of herpetic encephalitis. Pharmacol Rep. 2011;63(5):1203–9. doi: 10.1016/s1734-1140(11)70640-5 [DOI] [PubMed] [Google Scholar]
- 174.Blom K, Braun M, Pakalniene J, Lunemann S, Enqvist M, Dailidyte L, et al. NK Cell Responses to Human Tick-Borne Encephalitis Virus Infection. J Immunol. 2016;197(7):2762–71. doi: 10.4049/jimmunol.1600950 [DOI] [PubMed] [Google Scholar]
- 175.Babu GN, Kalita J, Misra UK. Inflammatory markers in the patients of Japanese encephalitis. Neurol Res. 2006;28(2):190–2. doi: 10.1179/016164106X98062 [DOI] [PubMed] [Google Scholar]
- 176.Aurelius E, Andersson B, Forsgren M, Sköldenberg B, Strannegård Ö. Cytokines and other markers of intrathecal immune response in patients with herpes simplex encephalitis. J Infect Dis. 1994;170(3):678–81. doi: 10.1093/infdis/170.3.678 [DOI] [PubMed] [Google Scholar]
- 177.Asaoka K, Shoji H, Nishizaka S, Ayabe M, Abe T, Ohori N, et al. Non-herpetic acute limbic encephalitis: Cerebrospinal fluid cytokines and magnetic resonance imaging findings. Intern Med. 2004;43(1):42–8. doi: 10.2169/internalmedicine.43.42 [DOI] [PubMed] [Google Scholar]
- 178.Ai P, Zhang X, Xie Z, Liu G, Liu X, Pan S, et al. The HMGB1 is increased in CSF of patients with an Anti-NMDAR encephalitis. Acta Neurol Scand. 2018;137(2):277–82. doi: 10.1111/ane.12850 [DOI] [PubMed] [Google Scholar]
- 179.Zajkowska J, Moniuszko-Malinowska A, Pancewicz SA, Muszynska-Mazur A, Kondrusik M, Grygorczuk S, et al. Evaluation of CXCL10, CXCL11, CXCL12 and CXCL13 chemokines in serum and cerebrospinal fluid in patients with tick borne encephalitis (TBE). Advances in Medical Sciences. 2011;56(2):311–7. doi: 10.2478/v10039-011-0033-z [DOI] [PubMed] [Google Scholar]
- 180.Maric LS, Lepej SZ, Gorenec L, Grgic I, Trkulja V, Rode OD, et al. Chemokines CXCL10, CXCL11, and CXCL13 in acute disseminated encephalomyelitis, non-polio enterovirus aseptic meningitis, and neuroborreliosis: CXCL10 as initial discriminator in diagnostic algorithm? Neurol Sci. 2018;39(3):471–9. doi: 10.1007/s10072-017-3227-8 [DOI] [PubMed] [Google Scholar]
- 181.Lind L, Eriksson K, Grahn A. Chemokines and matrix metalloproteinases in cerebrospinal fluid of patients with central nervous system complications caused by varicella-zoster virus. J Neuroinflamm. 2019;16:11. doi: 10.1186/s12974-019-1428-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pilotto A, Masciocchi S, Volonghi I, De Giuli V, Caprioli F, Mariotto S, et al. SARS-CoV-2 encephalitis is a cytokine release syndrome: evidences from cerebrospinal fluid analyses. Clin Infect Dis. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xie J, Guo CX, Zhang XL, Chen JJ, Huang X, Yu CB. CHANGES IN IFN-Gamma AND IL-4 LEVELS IN THE SERUM AND CSF OF PATIENTS WITH SUPPURATIVE MENINGITIS AND VIRAL ENCEPHALITIS. Acta Medica Mediterr. 2021;37(1):213–7. [Google Scholar]
- 184.Zou C, Pei SS, Yan W, Lu QB, Zhong XM, Chen Q, et al. Cerebrospinal Fluid Osteopontin and Inflammation-Associated Cytokines in Patients With Anti-N-Methyl-D-Aspartate Receptor Encephalitis. Frontiers in Neurology. 2020;11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Huang CN, Tian XB, Jiang SM, Chang SH, Wang N, Liu MQ, et al. Comparisons Between Infectious and Autoimmune Encephalitis: Clinical Signs, Biochemistry, Blood Counts, and Imaging Findings. Neuropsychiatr Dis Treat. 2020;16:2649–60. doi: 10.2147/NDT.S274487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mailles A, Stahl JP, Bloch KC. Update and new insights in encephalitis. Clin Microbiol Infect. 2017;23(9):607–13. doi: 10.1016/j.cmi.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 187.Turtle L, Solomon T. Encephalitis, Viral. In: Aminoff MJ, Daroff RB, editors. Encyclopedia of the Neurological Sciences (Second Edition). Oxford: Academic Press; 2014. p. 20–4. [Google Scholar]
- 188.Wang Y, Jia J, Wang Y, Li F, Song X, Qin S, et al. Roles of HSV-1 infection-induced microglial immune responses in CNS diseases: friends or foes? Critical reviews in microbiology. 2019;45(5–6):581–94. doi: 10.1080/1040841X.2019.1660615 [DOI] [PubMed] [Google Scholar]
- 189.Menendez CM, Carr DJ. Defining nervous system susceptibility during acute and latent herpes simplex virus-1 infection. J Neuroimmunol. 2017;308:43–9. doi: 10.1016/j.jneuroim.2017.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sørensen LN, Reinert LS, Malmgaard L, Bartholdy C, Thomsen AR, Paludan SR. TLR2 and TLR9 synergistically control herpes simplex virus infection in the brain. The Journal of Immunology. 2008;181(12):8604–12. doi: 10.4049/jimmunol.181.12.8604 [DOI] [PubMed] [Google Scholar]
- 191.Tognarelli EI, Palomino TF, Corrales N, Bueno SM, Kalergis AM, González PA. Herpes simplex virus evasion of early host antiviral responses. Frontiers in cellular and infection microbiology. 2019;9:127. doi: 10.3389/fcimb.2019.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhou Y, Ye L, Wan Q, Zhou L, Wang X, Li J, et al. Activation of Toll‐like receptors inhibits herpes simplex virus‐1 infection of human neuronal cells. Journal of neuroscience research. 2009;87(13):2916–25. doi: 10.1002/jnr.22110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mancini M, Vidal SM. Insights into the pathogenesis of herpes simplex encephalitis from mouse models. Mammalian genome. 2018;29(7):425–45. doi: 10.1007/s00335-018-9772-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Piret J, Boivin G. Innate immune response during herpes simplex virus encephalitis and development of immunomodulatory strategies. Reviews in medical virology. 2015;25(5):300–19. doi: 10.1002/rmv.1848 [DOI] [PubMed] [Google Scholar]
- 195.Conrady CD, Drevets DA, Carr DJ. Herpes simplex type I (HSV-1) infection of the nervous system: is an immune response a good thing? Journal of neuroimmunology. 2010;220(1–2):1–9. doi: 10.1016/j.jneuroim.2009.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chew T, Taylor KE, Mossman KL. Innate and adaptive immune responses to herpes simplex virus. Viruses. 2009;1(3):979–1002. doi: 10.3390/v1030979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Verzosa AL, McGeever LA, Bhark S-J, Delgado T, Salazar N, Sanchez EL. Herpes Simplex Virus 1 Infection of Neuronal and Non-Neuronal Cells Elicits Specific Innate Immune Responses and Immune Evasion Mechanisms. Frontiers in Immunology. 2021;12. doi: 10.3389/fimmu.2021.644664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Horan KA, Hansen K, Jakobsen MR, Holm CK, Søby S, Unterholzner L, et al. Proteasomal degradation of herpes simplex virus capsids in macrophages releases DNA to the cytosol for recognition by DNA sensors. The Journal of Immunology. 2013;190(5):2311–9. doi: 10.4049/jimmunol.1202749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG, Keane RW. Pattern recognition receptors and central nervous system repair. Experimental neurology. 2014;258:5–16. doi: 10.1016/j.expneurol.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang J, Cheng Y, Ma Y, Wu R, Xu Y, Yang S, et al. Cytokines and chemokines expression pattern in herpes simplex virus type-1 encephalitis. Neurosci Lett. 2021;763:136170. doi: 10.1016/j.neulet.2021.136170 [DOI] [PubMed] [Google Scholar]
- 201.Miyazaki D, Haruki T, Takeda S, Sasaki S-i, Yakura K, Terasaka Y, et al. Herpes simplex virus type 1–induced transcriptional networks of corneal endothelial cells indicate antigen presentation function. Investigative ophthalmology & visual science. 2011;52(7):4282–93. doi: 10.1167/iovs.10-6911 [DOI] [PubMed] [Google Scholar]
- 202.Li H, Zhang J, Kumar A, Zheng M, Atherton SS, Yu FSX. Herpes simplex virus 1 infection induces the expression of proinflammatory cytokines, interferons and TLR7 in human corneal epithelial cells. Immunology. 2006;117(2):167–76. doi: 10.1111/j.1365-2567.2005.02275.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: from physiopathology to therapy. Journal of hepatology. 2016;64(6):1403–15. doi: 10.1016/j.jhep.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 204.Shi L, Yin F, Xin X, Mao S, Hu P, Zhao C, et al. Astragalus polysaccharide protects astrocytes from being infected by HSV-1 through TLR3/NF-B signaling pathway. Evidence-Based Complementary and Alternative Medicine. 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Song J-D, Liu X-L, Chen D-L, Zou X-H, Wang M, Qu J-G, et al. Human adenovirus type 41 possesses different amount of short and long fibers in the virion. Virology. 2012;432(2):336–42. doi: 10.1016/j.virol.2012.05.020 [DOI] [PubMed] [Google Scholar]
- 206.Lannes N, Summerfield A, Filgueira L. Regulation of inflammation in Japanese encephalitis. Journal of neuroinflammation. 2017;14(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chen C-J, Ou Y-C, Lin S-Y, Raung S-L, Liao S-L, Lai C-Y, et al. Glial activation involvement in neuronal death by Japanese encephalitis virus infection. Journal of General Virology. 2010;91(4):1028–37. doi: 10.1099/vir.0.013565-0 [DOI] [PubMed] [Google Scholar]
- 208.Lannes N, Neuhaus V, Scolari B, Kharoubi-Hess S, Walch M, Summerfield A, et al. Interactions of human microglia cells with Japanese encephalitis virus. Virology journal. 2017;14(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Burke DS, Morrill JC. Levels of interferon in the plasma and cerebrospinal fluid of patients with acute Japanese encephalitis. The Journal of infectious diseases. 1987;155(4):797–9. doi: 10.1093/infdis/155.4.797 [DOI] [PubMed] [Google Scholar]
- 210.Leyssen P, Paeshuyse J, Charlier N, Van Lommel A, Drosten C, De Clercq E, et al. Impact of direct virus-induced neuronal dysfunction and immunological damage on the progression of flavivirus (Modoc) encephalitis in a murine model. Journal of neurovirology. 2003;9(1):69–78. doi: 10.1080/13550280390173319 [DOI] [PubMed] [Google Scholar]
- 211.Wang Y, Lobigs M, Lee E, Müllbacher A. CD8+ T Cells Mediate Recovery andImmunopathology in West Nile VirusEncephalitis. Journal of virology. 2003;77(24):13323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. The Lancet Neurology. 2016;15(4):391–404. doi: 10.1016/S1474-4422(15)00401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dalmau J, Graus F. Antibody-mediated encephalitis. New England Journal of Medicine. 2018;378(9):840–51. doi: 10.1056/NEJMra1708712 [DOI] [PubMed] [Google Scholar]
- 214.Heine J, Prüss H, Bartsch T, Ploner C, Paul F, Finke C. Imaging of autoimmune encephalitis–Relevance for clinical practice and hippocampal function. Neuroscience. 2015;309:68–83. doi: 10.1016/j.neuroscience.2015.05.037 [DOI] [PubMed] [Google Scholar]
- 215.Singh TD, Fugate JE, Rabinstein AA. The spectrum of acute encephalitis: causes, management, and predictors of outcome. Neurology. 2015;84(4):359–66. doi: 10.1212/WNL.0000000000001190 [DOI] [PubMed] [Google Scholar]
- 216.Wesselingh R, Butzkueven H, Buzzard K, Tarlinton D, O’Brien TJ, Monif M. Innate Immunity in the Central Nervous System: A Missing Piece of the Autoimmune Encephalitis Puzzle? Front Immunol. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Deng B, Liu X-N, Li X, Zhang X, Quan C, Chen X-J. Raised cerebrospinal fluid BAFF and APRIL levels in anti-N-methyl-d-aspartate receptor encephalitis: correlation with clinical outcome. Journal of neuroimmunology. 2017;305:84–91. doi: 10.1016/j.jneuroim.2017.01.012 [DOI] [PubMed] [Google Scholar]
- 218.Kimura A, Yoshikura N, Koumura A, Hayashi Y, Inuzuka T. B-cell-activating factor belonging to the tumor necrosis factor family (BAFF) and a proliferation-inducing ligand (APRIL) levels in cerebrospinal fluid of patients with meningoencephalitis. Journal of the neurological sciences. 2015;352(1–2):79–83. doi: 10.1016/j.jns.2015.03.036 [DOI] [PubMed] [Google Scholar]
- 219.Jun JS, Lee ST, Kim R, Chu K, Lee SK. Tocilizumab treatment for new onset refractory status epilepticus. Annals of neurology. 2018;84(6):940–5. doi: 10.1002/ana.25374 [DOI] [PubMed] [Google Scholar]