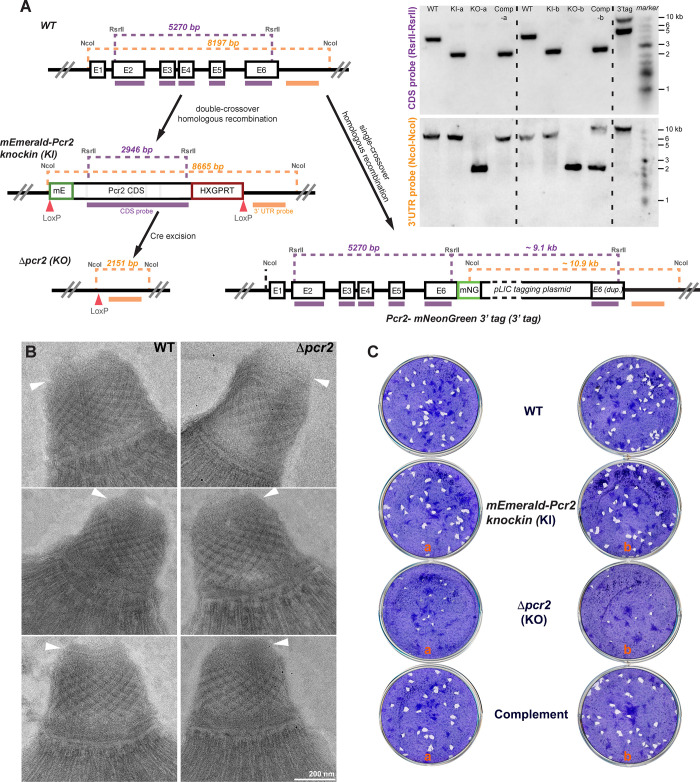
Fig 4. Generation of mEmeraldFP-Pcr2 knock-in and Δpcr2 parasites and assessment of their plaquing efficiency.
A. Left, schematic for generating mEmeraldFP-Pcr2 knock-in, Δpcr2 parasites, and the Pcr2-mNeonGreen 3’ endogenous tagged line, and Southern blotting strategy. The positions of the restriction sites, CDS probe (purple bar) and the probe annealing downstream of the pcr2 coding sequence (“3’ UTR probe”, orange bar) used in Southern blotting analysis and the corresponding DNA fragment sizes expected are shown (also see Materials and Methods for expected hybridization patterns). Right, Southern blotting analysis of the pcr2 locus in RHΔhxΔku80 (WT), mEmeraldFP-Pcr2 knock-in (KI), Δpcr2 (KO), complemented (Comp) parasites and the Pcr2-mNeonGreen 3’ tagged line (“3’tag”). The box representing the pLIC tagging plasmid is not drawn to scale. Two sets (a and b) of independently generated knock-in, knockout, and complemented lines were analyzed. B. EM examination of the apical complex in the RHΔhxΔku80 parental (WT, left column) and Δpcr2 (right column) parasites that had been incubated with the calcium ionophore A23187 (which induces conoid protrusion), followed by TX-100 treatment. Arrowheads: preconoidal rings. C. Plaques formed by RHΔhxΔku80 (WT), mEmeraldFP-Pcr2 knock-in (KI), Δpcr2 (KO), and complemented (Complement) parasite lines. Plaque assays for two independent sets of knock-in, knockout and complemented lines are shown. Nine days after inoculation, the cultures were fixed with 70% ethanol and then stained with crystal violet. “plaques” are cleared spaces where the HFF monolayers were destroyed by recurring cycles of parasite invasion, replication and egress.