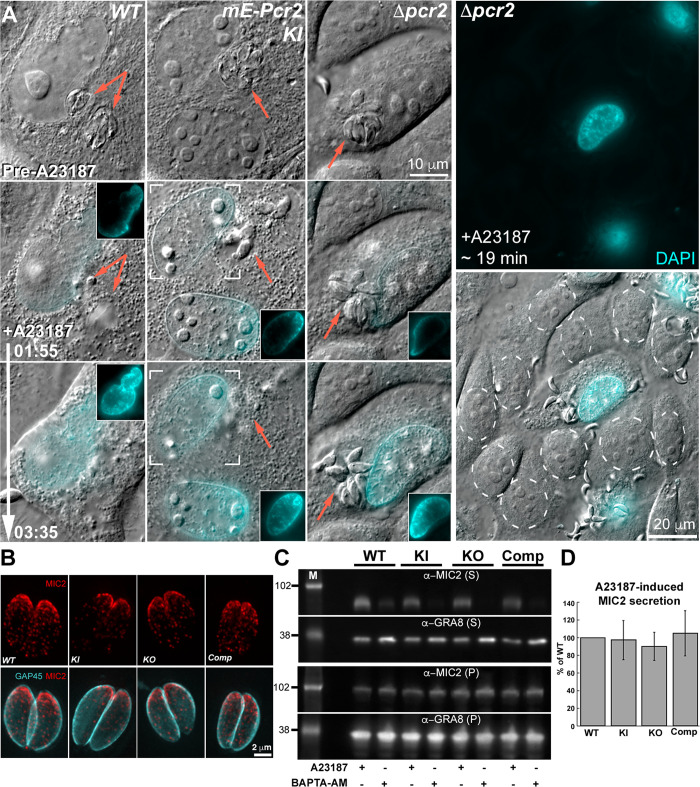
Fig 6. Calcium ionophore-induced micronemal secretion is not significantly affected in Δpcr2 parasites.
A. Images selected from time-lapse experiments of intracellular RHΔhxΔku80 (WT), mEmeraldFP-Pcr2 knock-in (mE-Pcr2 KI), and knockout (Δpcr2) treated with 5 μM A23187 (also see S3 Video). The cell-impermeant DNA-binding dye, DAPI, was added to the medium to monitor the permeabilization of the host cell. Δpcr2 parasites are able to secrete effectors that lyse the host cell upon A23187 treatment, indicated by DAPI entering the host cell nucleus and binding to DNA, as well as by the dramatic change in the morphology of the host cell (see S2 and S3 Videos). Insets are DAPI images of the nuclear region of the host cell shown at 0.5X. Brackets in the mE-Pcr2 KI panels indicate the host cell nucleus included in the insets. Contrast was adjusted so that the DAPI labeling at the rim of the nucleus is easily visible. The nuclei of uninfected fibroblasts (marked by dashed circles) remained unlabeled by DAPI ~19 min after A23187 treatment as shown in the larger field of view images in the right-hand column. B. Projections of deconvolved wide-field fluorescence images of intracellular WT, mE-Pcr2 KI, Δpcr2, and complemented (Comp) parasites labeled with a mouse anti-MIC2 (red), a rat anti-GAP45 (cyan) and corresponding secondary antibodies. C. Western blots of the secreted (supernatant, S) and unsecreted (pellet, P) fractions of WT, mE-Pcr2 KI, Δpcr2, and complemented (Comp) parasites after A23187 or BAPTA-AM (a calcium chelator; negative control) treatment. The blots were probed by antibodies against MIC2 and GRA8. M: molecular weight markers, the masses of which are indicated in kDa by the numbers on the left. D. Levels of MIC2 in the secreted fractions relative to that from the wild-type in 3 independent biological replicates. For each sample, the MIC2 secretion upon A23187 stimulation is normalized against GRA8 in the pellet from the same sample. Error bars: standard error.