Figure 2.
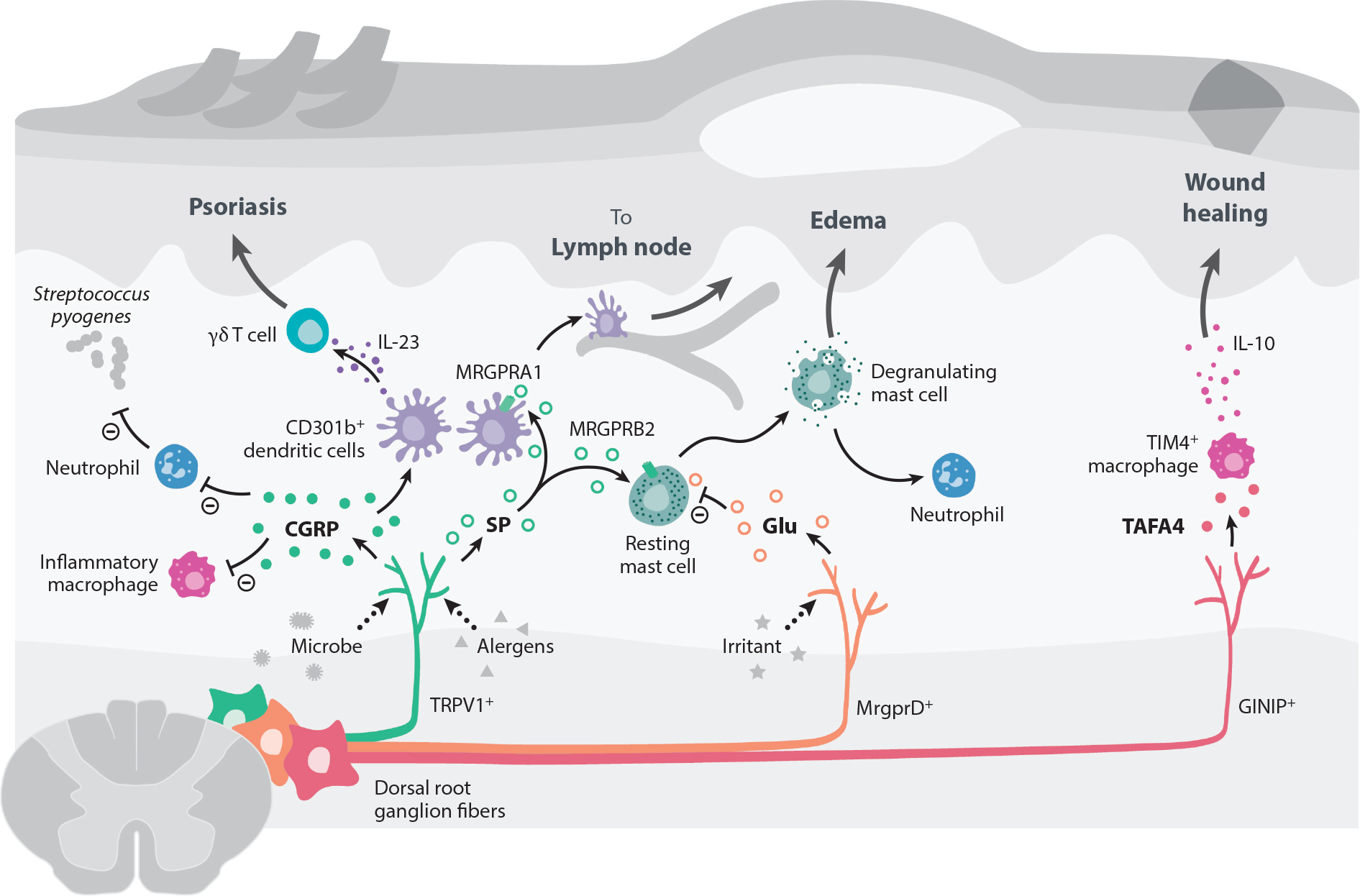
Peripheral neuroimmune interactions in the skin. Most of the skin is innervated by sensory fibers originating from dorsal root ganglia, which have been shown to communicate with skin-resident and infiltrating immune cells. In bacterial infections caused by Staphylococcus aureus or Streptococcus pyogenes, pore-forming toxins activate Nav1.8+ and TRPV1+ nociceptor neurons to release CGRP. CGRP can subsequently suppress macrophages from producing inflammatory cytokines like TNFα and decrease neutrophil recruitment and function. This inhibitory activity of CGRP leads to the loss of control of bacterial infection. TRPV1+ fibers can also be activated by Candida albicans to release CGRP during infection. CGRP acts on dermal CD301b+ DCs to produce IL-23, which promotes dermal γδ T cell proliferation and secretion of IL-17 to control fungal infection. However, this DC/γδ T cell/IL-17 circuit can also promote inflammation and pathology in a mouse model of psoriasis. Active proteases in allergens trigger TRPV1+ neurons to release SP, which acts on MRGPRA1 on DCs to promote their migration to draining lymph nodes and prime a Th2 immune response. SP release from TRPV1+ sensory neurons also trigger mast cell degranulation via binding MRGPRB2. The SP-MRGPRB2 mast cell axis drives tissue edema and neutrophil recruitment. By contrast, nonpeptidergic MRGPRD+ sensory neurons release glutamate to suppress mast cell degranulation. GINIP+ sensory fibers promote tissue healing by releasing the neuropeptide TAFA4 to promote release of the anti-inflammatory cytokine IL-10 by TIM4+ macrophages. Abbreviations: CGRP, calcitonin gene–related peptide; DC, dendritic cell; GINIP, Gαi-interacting protein; MRGPR, Mas-related G protein–coupled receptor; Nav, voltage-gated sodium channel; SP, substance P; TAFA4, TAFA chemokine like family member 4; Th2, T-helper type 2; TIM4, T cell immunoglobulin and mucin domain containing 4; TNFα, tumor necrosis factor alpha; TRPV1, transient receptor potential cation channel V member 1.
