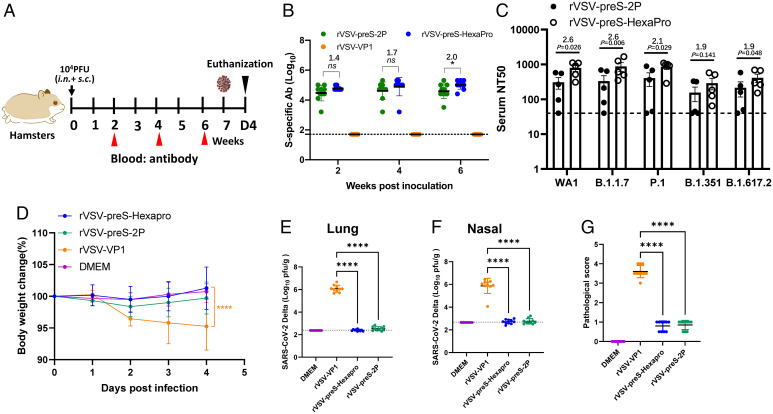
Fig. 8.
A single dose (104pfu) immunization of rVSV-preS-HexaPro and rVSV-preS-2P provides complete protection against challenge with SARS-CoV-2 Delta variant in hamsters. (A) Immunization schedule of the experiment. Twenty 4-wk-old SPF female hamsters were randomly divided into 4 groups (n =5 × 2, pooled from two independent experiments). Hamsters in groups 1–3 were immunized with 104 pfu of rVSV-VP1 (control virus), rVSV-preS-2P and rVSV-preS-HexaPro, respectively. Group 4 was inoculated with DMEM and served as normal control. All hamsters were immunized via a combination of subcutaneous and intranasal route (half subcutaneous and half intranasal). (B) Measurement of SARS-CoV-2 S-specific antibody by ELISA. Data are expressed as the GMT of ten hamsters in each group ± SD. (C) Comparison of neutralization efficiency of serum antibody against VoCs. Sera at week 4 were chosen for determining neutralizing antibody against each VoCs. (D) Body weight changes after challenge with SARS-CoV-2 Delta variant. The body weight for each mouse was expressed as percentage of body weight at the challenge day. The average body weight of 10 hamsters (n = 5 × 2) in each group was shown. (E) SARS-CoV-2 titer in lungs. (F) SARS-CoV-2 titer in nasal turbinate. At day 4 after challenge, and lungs and nasal turbinates were collected for virus titration. Viral titers are the GMT of 10 animals ± SD (G) Lung pathology score after challenge with SARS-CoV-2 Delta variant. Score 4 = extremely severe lung pathological changes; score 3 = severe lung pathological changes; score 2 = moderate lung pathological changes; score 1 = mild lung pathological changes; and score 0 = no pathological changes. Data were analyzed using two-way ANOVA and Student t test (*P < 0.05; ****P < 0.0001).