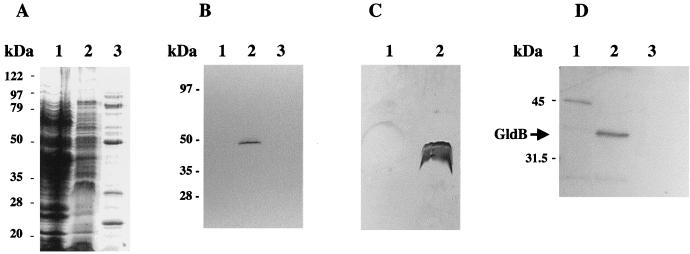
FIG. 6.
Localization of GldB to the Sarkosyl-soluble (cytoplasmic membrane) fraction of cells. Cells were disrupted and separated into soluble and membrane fractions. Membranes were fractionated further by differential solubilization in Sarkosyl as described in Materials and Methods. Equal amounts of each fraction were separated by SDS-PAGE and examined for total protein (A), cytochrome c (cytoplasmic membrane marker) (B), LPS (outer membrane marker) (C), and GldB protein (D). (A) Coomassie blue-stained gel to detect total protein. Lanes: 1, soluble (cytoplasmic and periplasmic) fraction; 2, Sarkosyl-soluble (cytoplasmic membrane) fraction; 3, Sarkosyl-insoluble (outer membrane) fraction. (B) Heme-stained gel to detect cytochrome c. Lanes: 1, soluble (cytoplasmic and periplasmic) fraction; 2, Sarkosyl-soluble (cytoplasmic membrane) fraction; 3, Sarkosyl-insoluble (outer membrane) fraction. (C) Silver-stained gel to detect LPS. Lanes: 1, Sarkosyl-soluble (cytoplasmic membrane) fraction; 2, Sarkosyl-insoluble (outer membrane) fraction. (D) Western blot analysis to detect GldB. Lanes: 1, soluble (cytoplasmic and periplasmic) fraction; 2, Sarkosyl-soluble (cytoplasmic membrane) fraction; 3, Sarkosyl-insoluble (outer membrane) fraction.