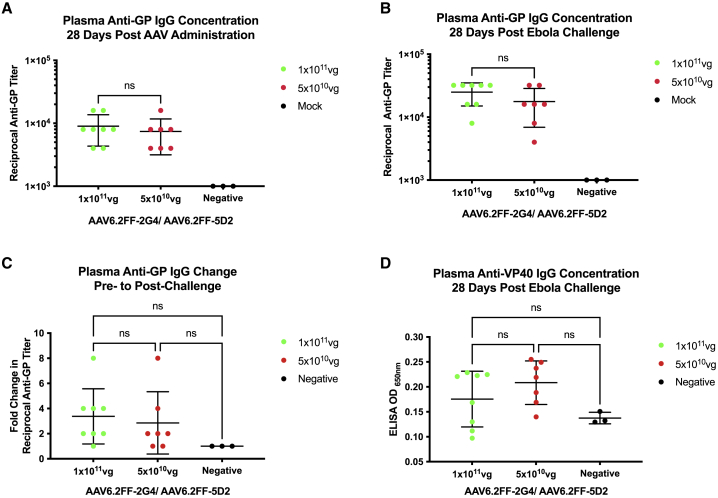
Figure 2.
EBOV GP reciprocal antibody titers before and after challenge from mice that survived MA-EBOV challenge
Sera from the surviving mice in the AAV6.2FF-2G4/AAV6.2FF-5D2 dose reduction experiment (1 × 1011 vg, n = 8, and 5 × 1010 vg, n = 7) were analyzed by EBOV GP ELISA (A) 28 days after AAV administration but before challenge and (B) 28 days after challenge. (C) The fold change in pre- to post-challenge reciprocal anti-GP titers observed at each dose. (D) 28 days post-challenge serum samples were analyzed at a 1:100 dilution by EBOV VP40 ELISA; negative controls were serum samples from naive, untreated mice separate from the challenge studies. A one-way ANOVA with Tukey’s multiple comparisons test revealed no significant differences between groups. All error bars represent the standard deviation of the mean.