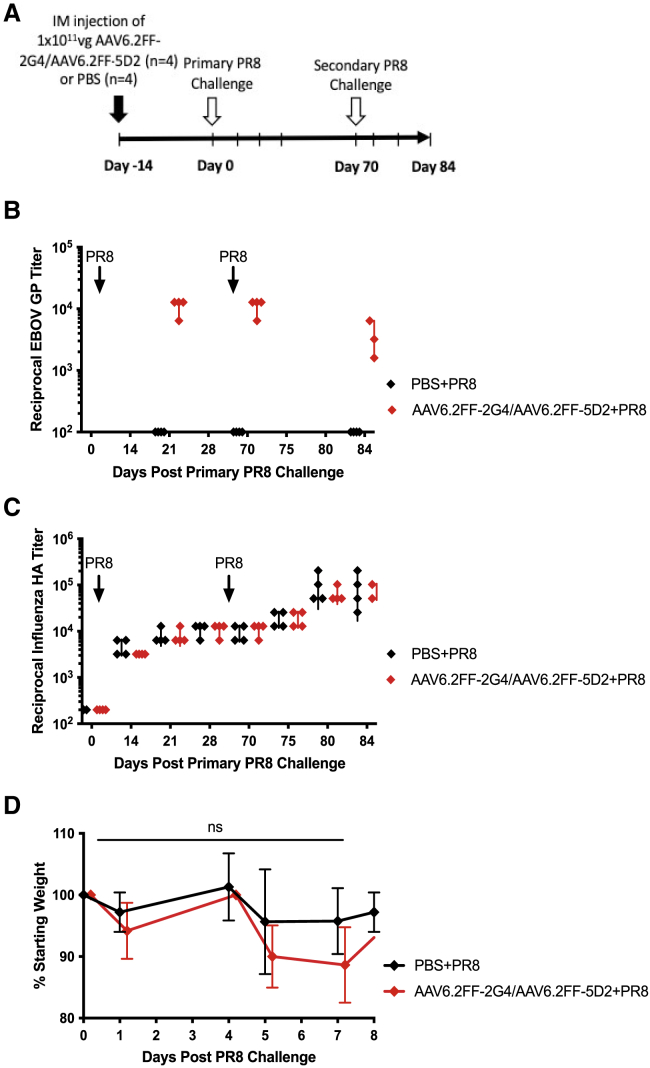
Figure 3.
Endogenous humoral response to influenza A virus in the context of protective 2G4/5D2 antibody titers
(A) Schematic of experimental design. BALB/c mice (n = 4/group) were administered 1 × 1011 vg of AAV6.2FF-2G4/AAV6.2FF-5D2 IM 14 days prior to primary sub-lethal exposure to 600 HA units of influenza A virus (strain PR8) by IP injection. (B) Reciprocal EBOV GP titers from serum samples from AAV6.2FF-2G4/AAV6.2FF-5D2- or PBS-treated groups. (C) Reciprocal HA titers following primary and secondary exposure to 600 HA units of influenza A virus (strain PR8) in mice treated with AAV6.2FF-2G4/AAV6.2FF-5D2 or PBS. (D) Average weight change in mice following primary influenza A virus. A two-way ANOVA and Šídák’s test for multiple comparisons was conducted to compare weights between the two treatment groups. All error bars represent the standard deviation of the mean.