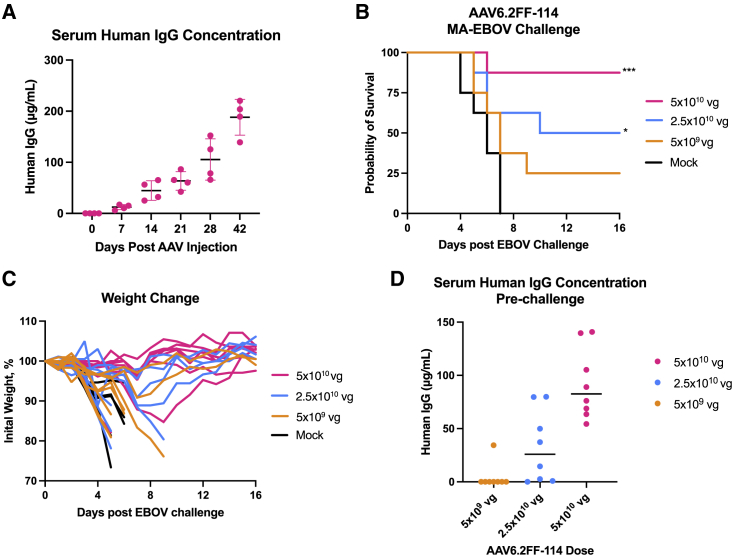
Figure 5.
AAV6.2FF-114 mediates moderate protection from EBOV challenge
(A) Expression kinetics of mAb114 measured as human IgG in the serum of BALB/c mice (n = 4) that received 5 × 1010 vg of AAV6.2FF-114 IM and were monitored over the course of 42 days. BALB/c mice (n = 8/group) were administered various doses of AAV6.2FF-114 via IM administration, while controls were administered 5 × 1010 vg of AAV6.2FF expressing an irrelevant influenza mAb, FluA-20. At 28 days after AAV administration, mice were challenged with 1000xLD50 MA-EBOV and monitored for (B) survival and (C) weight loss (individual mice plotted). (D) Serum concentrations of human IgG were quantified 1 day prior to challenge. Survival of treated groups was compared with the mock group using the Mantel-Cox log rank test. ∗∗∗p < 0.001 and ∗p < 0.05 for mice treated with 5 × 1010 vg and 5 × 109 vg, respectively, of AAV6.2FF-114 during the 1000xLD50 MA-EBOV challenge. All error bars represent the standard deviation of the mean.