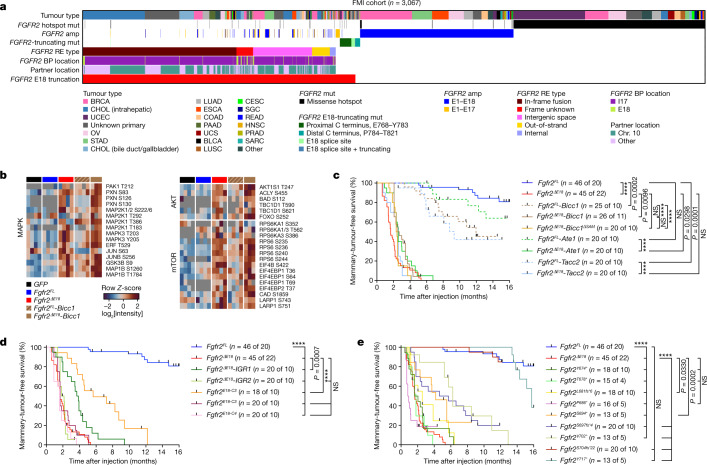
Fig. 2. Human FGFR2 E18-truncating alterations are oncogenic drivers in mice.
a, Analysis of 3,067 samples (1.23% incidence) containing FGFR2-I17/E18 in-frame fusions (n = 757, 0.30% incidence), frame unknown REs (n = 82, 0.03% incidence), intergenic space REs (n = 291, 0.12% incidence), out-of-strand REs (n = 88, 0.04% incidence), internal REs (n = 29, 0.01% incidence), FGFR2-E18 splice-site mutations (mut; n = 21, 0.01% incidence), E18-truncating nonsense and frameshift mutations (proximal, n = 59, 0.02% incidence; distal, n = 23, 0.01% incidence), FGFR2-E1–E17 partial amplifications (amp; n = 73, 0.03% incidence), E1–E18 full-length amplifications (n = 838, 0.34% incidence), and/or FGFR2 missense hotspot mutations affecting Ser252, Cys382, Asn549 or Lys659 (n = 978, 0.39% incidence) found in 249,570 pan-cancer diagnostic panel-seq profiles from FMI. BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL, cholangiocarcinoma; chr, chromosome; COAD, colon adenocarcinoma; ESCA, oesophageal carcinoma; HNSC, head and neck squamous cell carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PRAD, prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC, sarcoma; SGC, salivary gland carcinoma; STAD, stomach adenocarcinoma; UCEC, uterine corpus endometrial carcinoma; UCS, uterus carcinosarcoma. b, Global phosphoproteomic analysis of NMuMG cells expressing GFP or the indicated Fgfr2 variants. Groups were compared in a pairwise manner using the robust kinase activity inference (RoKAI) tool, including two-tailed hypothesis testing on Z-scores and false-discovery rate (FDR) multiple-testing correction using the Benjamini–Hochberg method. Group-comparison fold change (FC) values of −1.5 ≥ FC ≥ 1.5 and P < 0.05 were considered. The heatmaps show phosphosites subselected from the RoKAI output and grouped into the indicated signalling pathways guided by RoKAI as colour-coded row Z-scores calculated from log2-transformed intensity values. c–e, Kaplan–Meier curves showing mammary-tumour-free survival of female mice intraductally injected with lentiviruses encoding the indicated Fgfr2 variants. Cohort counts (n) are injected mammary glands (MGs) per number of mice. The Fgfr2FL and Fgfr2ΔE18 curves in c are duplicated in d and e. P values were calculated using log-rank (Mantel–Cox) tests; ****P < 0.0001; NS, not significant (P ≥ 0.05).