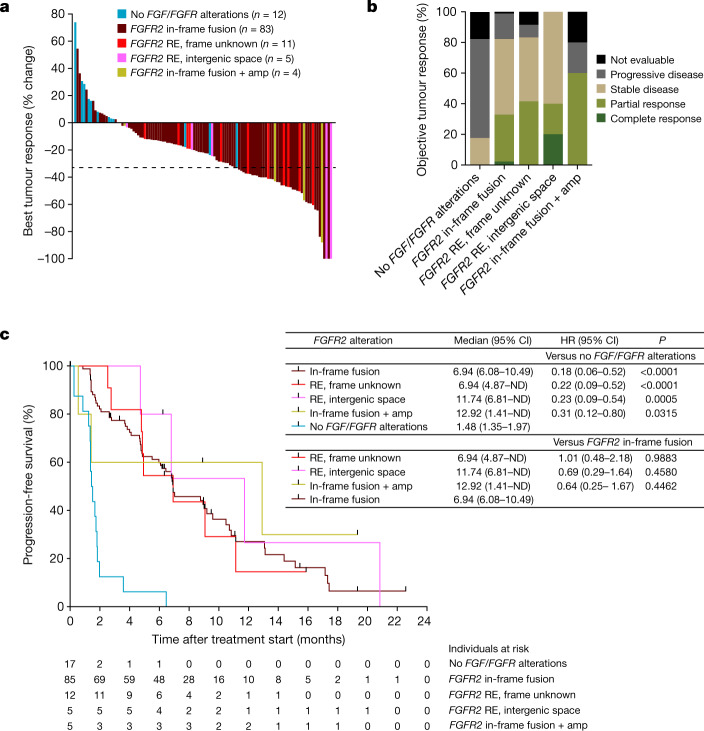
Fig. 5. Patients with cholangiocarcinoma respond to FGFR-targeted therapy irrespective of FGFR2 RE type.
a, Centrally assessed best percentage change from the baseline in target lesion size of 115 (92%) of 125 individual patients with cholangiocarcinoma treated with pemigatinib, who had post-baseline scans. Data are from the FIGHT-202 study9 and the coloured bars indicate FGFR2-I17/E18 RE types and FGFR2 amplification status as diagnosed by FoundationOne. b, Objective tumour responses observed in the FIGHT-202 study assessed according to the Response Evaluation Criteria in Solid Tumours v.1.1 (RECIST 1.1) and grouped according to FGFR2 RE types/amplification status. No FGF/FGFR alterations (n = 17), FGFR2 in-frame fusion (n = 85), frame unknown RE (n = 12), intergenic space RE (n = 5), in-frame fusion + amplification (n = 5). ‘Not evaluable’ indicates that the patient was not evaluable for response using RECIST. c, Kaplan–Meier analysis of the progression-free survival of patients with cholangiocarcinoma treated with pemigatinib from the FIGHT-202 study and grouped according to FGFR2 RE types/amplification status. Data are median ± 95% CI for each cohort, and log-rank hazard ratios (HR) ± 95% CI for the indicated comparisons are shown. P values were calculated using log-rank (Mantel–Cox) tests. ND, not defined.