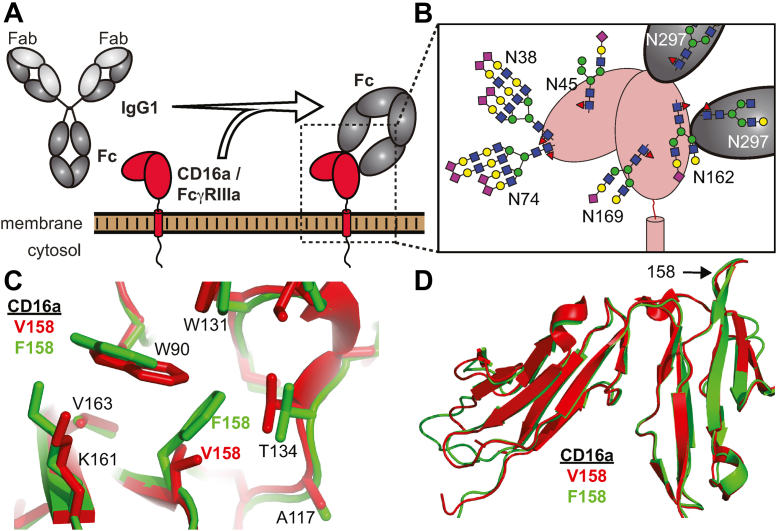
Figure 1.
Two common receptor allotypes in the human genome differ by a single amino acid substitution at position 158 in the extracellular antibody-binding domain.A, CD16a/Fc γ receptor (FcγR) IIIa binds the crystallizable fragment (Fc) of immunoglobulin G1 (IgG1). B, CD16a is heavily N-glycosylated with five N-glycans. The predominant glycoforms identified on primary human natural killer cells are shown as cartoons and scaled to the appropriate size. Fc is likewise glycosylated at N297. An overlay of the CD16a V158 structure (red, pdb 5vu0) with an AlphaFold model of CD16a F158 (green) shows residues surrounding the 158 position (C) and the global structural similarity (D).