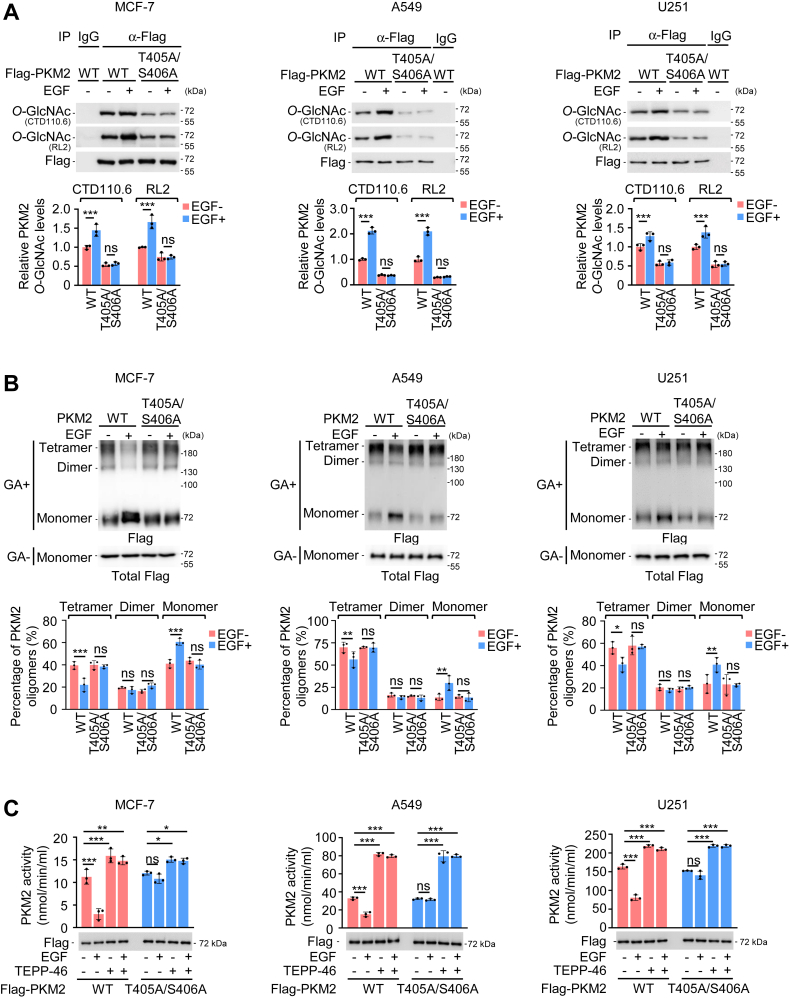
Figure 1.
EGF induces PKM2 detetramerization and reduced its activity.A, EGF upregulates PKM2 T405/S406 O-GlcNAcylation. (top) Transfection of Flag-tagged PKM2WT or PKM2T405A/S406A along with or without concomitant EGF treatment (100 ng/ml for 1 h) was conducted in MCF-7, A549, or U251 cells. Following Flag immunoprecipitation (IP), samples were analyzed by western blotting (WB) with antibodies against Flag and O-GlcNAcylation (CTD110.6 and RL2), respectively. IgG is the negative control. (bottom) Relative protein levels of IP. PKM2 O-GlcNAc was normalized to immunoprecipitated Flag-PKM2. Quantification shows mean ± SD (n = 3) with significance determined by two-way ANOVA, ∗∗∗p < 0.001, ns, nonsignificant. B, distribution of PKM2 oligomers. (top) MCF-7, A549, and U251 cells transfected with Flag-tagged PKM2WT or PKM2T405A/S406A for 24 h were incubated in the presence or absence of EGF (100 ng/ml for 1 h) and subsequently analyzed by WB. GA, glutaraldehyde. (bottom) Quantification of PKM2 oligomers. Tetramers, dimers, and monomers were normalized according to total Flag-PKM2 in each group. Data represent mean ± SD (n = 3) with significance determined by two-way ANOVA, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p <0.001, ns, nonsignificant. C, the effect of EGF on PKM2 activity. MCF-7, A549, and U251 cells were transfected with Flag-tagged PKM2WT or PKM2T405A/S406A for 24 h in the presence or absence of TEPP-46 (100 μM, 24 h). Subsequently, they were treated with or without EGF (100 ng/ml) for 1 h prior to the analysis of PKM2 enzymatic activity. Data represent mean ± SD (n = 3) with significance determined by one-way ANOVA, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ns, nonsignificant. EGF, epidermal growth factor.