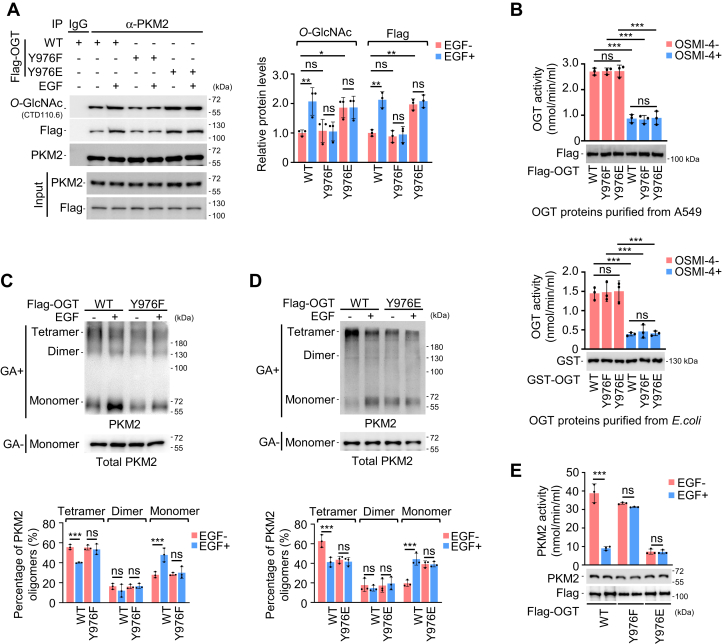
Figure 5.
OGT Y976 phosphorylation is important for EGF-induced O-GlcNAcylation on PKM2.A, impact from mutations in OGT on PKM2 O-GlcNAcylation. (left) A549 cells transfected with Flag-tagged OGTWT, OGTY976F (unphosphorylatable), or OGTY976E (unphosphorylatable) were incubated with or without EGF (100 ng/ml) for 1 h and then subjected to IP analysis for OGT-PKM2 interaction and PKM2 O-GlcNAcylation. (right) Relative protein levels of IP. PKM2 O-GlcNAc and Flag-OGT were normalized to PKM2. Quantification shows mean ± SD (n = 3) with significance determined by two-way ANOVA, ∗p < 0.05, ∗∗p < 0.01, ns, nonsignificant. B, activity assay for OGT. Flag-tagged OGTWT, OGTY976F, and OGTY976E proteins purified from A549 cells (top), and GST-tagged OGT proteins purified from E. coli (bottom) were evaluated for their enzymatic activity in the presence or absence of OSMI-4 (3 μM). Reduction in UDP-GlcNAc is being measured and calculated to indicate OGT activity. Data represent mean ± SD (n = 3) with significance determined by two-way ANOVA, ns, nonsignificant. C and D, assay for PKM2 oligomers with OGT mutants. (top) The whole cell lysates from A549 cells treated as (A) were crosslinked and analyzed by WB. (bottom) Quantification of PKM2 tetramers, dimers, and monomers was based on the normalization to total PKM2. Data represent mean ± SD (n = 3) with significance determined by two-way ANOVA, ∗∗∗p < 0.001, ns, nonsignificant. E, Assay for PKM2 activity with OGT mutants. PKM2 isolated from cells treated as (A) were assayed for enzymatic activity. Data represent mean ± SD (n = 3) with significance determined by two-way ANOVA, ∗∗∗p < 0.001, ns, nonsignificant. EGF, epidermal growth factor; IP, immunoprecipitation; OGT, O-GlcNAc transferase.