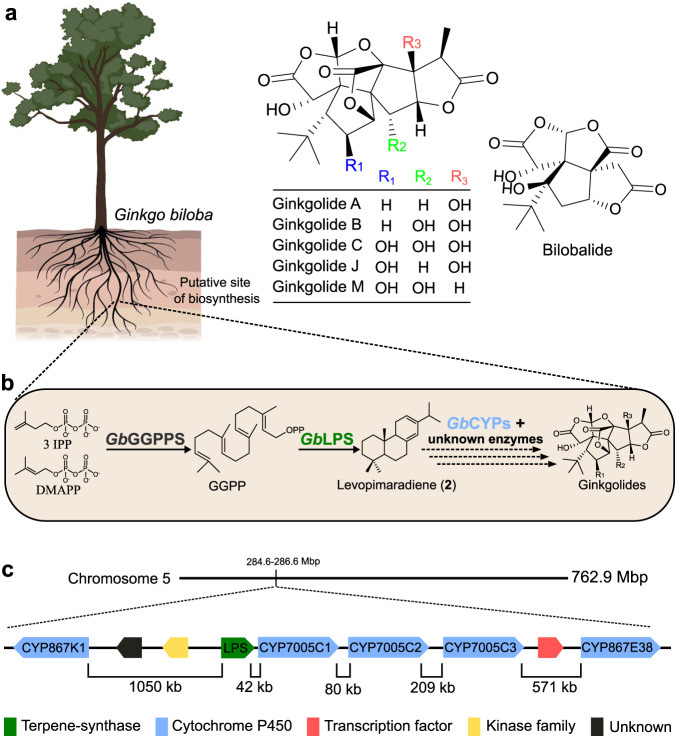
Fig. 1. Biosynthesis of ginkgolides.
a Chemical structure of ginkgolides (diterpenoids) and bilobalide (sesquiterpenoid). The tree image has been created by BioRender.com (2021). b Ginkgolide biosynthesis is suggested to take place in the roots of the tree, as indicated by the dashed lines. Geranylgeranyl pyrophosphate synthase (GbGGPPS) is producing geranylgeranyl pyrophosphate (GGPP), which initiates the biosynthesis of all diterpenoids. Next, levopimaradiene synthase (GbLPS) converts GGPP to diterpene hydrocarbon levopimaradiene, which is the hypothesized precursor of ginkgolides. It has been proposed that levopimaradiene is oxygenated by cytochrome P450s (CYPs) en route to ginkgolides. Further rearrangements in the carbon skeleton may be catalyzed by CYPs in connection with oxidation or by yet unknown enzymes. c Organization of the identified biosynthetic gene cluster (BGC) on chromosome 5. The GbLPS gene is located in close proximity to five CYP genes. Genes coding for a putative kinase, a GTE10-like transcription factor, and an unknown protein are also present (indicated by different colored boxes). Mbp million base pairs, kb kilo base pairs.