Fig. 2. Ginkgosinoic acid A (7) is an intermediate from levopimaradiene toward ginkgolides.
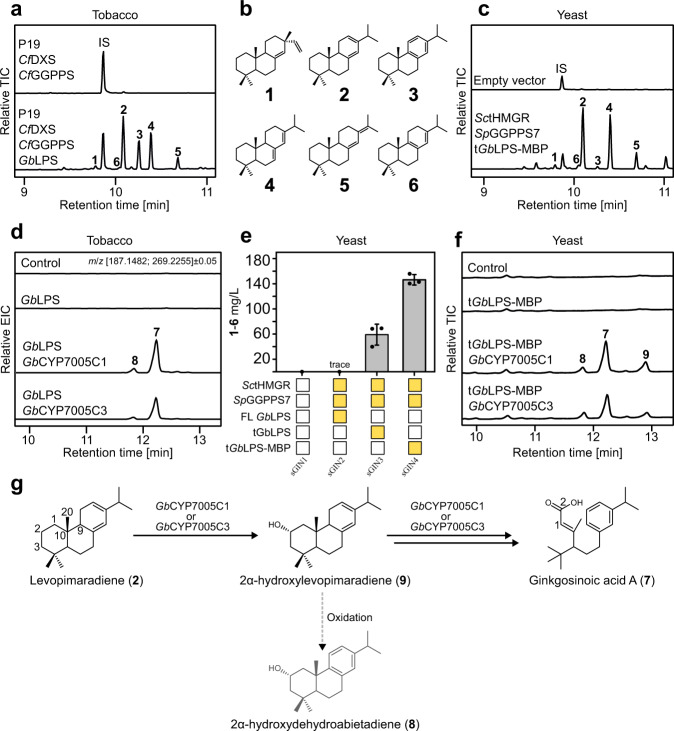
a GC–MS chromatograms of hexane extracts of N. benthamiana (tobacco) leaves transiently expressing CfDXS (C. forskohlii 1-deoxy-d-xylulose 5-phosphate synthase) and CfGGPPS (C. forskohlii geranylgeranyl pyrophosphate synthase) with or without GbLPS (G. biloba levopimaradiene synthase). In all samples, the P19 silencing suppressor is co-expressed. IS internal standard. b Chemical structure of compounds 1–6 identified as GbLPS products (Supplementary Fig. 1). c GC–MS chromatograms of hexane extracts of S. cerevisiae cells carrying an empty vector or expressing SctHMGR (a truncated version of yeast 3-hydroxy-3-methylglutaryl-coenzyme A reductase), SpGGPPS7 (Synechococcus sp. geranylgeranyl pyrophosphate synthase) with a truncated version of codon-optimized GbLPS fused with maltose-binding protein (MBP) for optimal expression. IS internal standard. d Transient co-expression of GbLPS together with either GbCYP7005C1 or GbCYP7005C3 in tobacco leaves. The co-expression led to the accumulation of compounds 7 and 8, as shown on extracted ion chromatograms (EICs) from LC–HRMS analysis of methanolic extracts. e Optimization of GbLPS efficiency in yeast by truncation of the plastid transit peptide and fusion to MBP at the C-terminal end, as monitored by GC-MS-based quantification. Error bars indicate a standard deviation of n = 3 biological independent replicates. f Formation of compounds 7–9 in yeast cells expressing GbCYP7005C1 or GbCYP7005C3 together with GbPOR2, SpGGPPS7, SctHMGR and GbLPS. The chromatograms show total ion chromatograms (TICs) from LC–HRMS analysis of methanolic yeast extracts. g Proposal for the reactions catalyzed by GbCYP7005C1 or GbCYP7005C3. The chemical structures of 7–9 were elucidated using NMR. Compounds 8 and 9 were established as side-product and intermediate, respectively, using feeding studies (Supplementary Fig. 5a, c, e). The numbering of carbon atoms follows the standard numbering of levopimaradiene. Source data are provided as a Source Data file.