Figure 3.
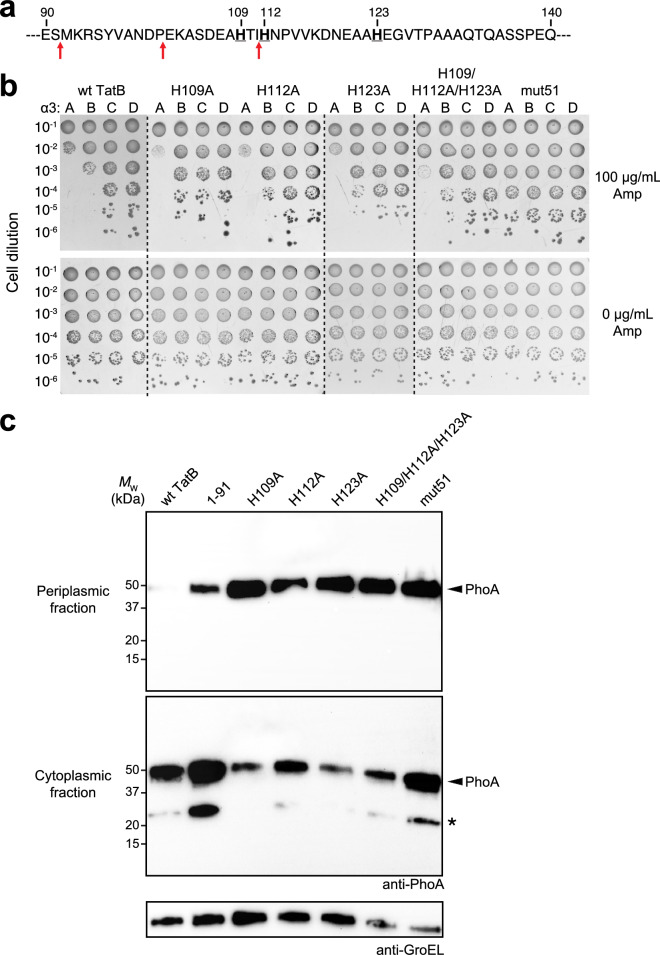
Histidine patch in membrane-extrinsic domain of TatB regulates folding QC. (a) Sequence of residues 90–140 of the membrane-extrinsic domain of TatB. Bold underline font denotes histidines at positions 109, 112, and 123; red arrows indicate QC-relevant truncation sites. (b) Serially diluted DADE cells co-expressing spTorA-α3-Bla chimeras (A, B, C or D) along with a Tat operon plasmid encoding wildtype (wt) TatB, one of the TatB variants, or the mut51 translocase were spotted on LB-agar plates containing either 100 μg/mL Amp or 25 μg/mL Cam (0 μg/mL Amp). Dashed lines denote different LB-agar plates that were all generated and imaged at the same time. (c) Western blot analysis of cytoplasmic and periplasmic fractions prepared from DADE cells co-expressing Tat-targeted PhoA from pTorA-AP along with either wt TatABC, mut51, or one of the histidine mutants as indicated. An equivalent number of cells was loaded in each lane. PhoA was probed with anti-PhoA antibody while anti-GroEL antibody confirmed equivalent loading in each lane. Asterisk indicates spTorA-PhoA breakdown product. Molecular weight (MW) markers are indicated on the left. Results are representative of three biological replicates. See Supplementary Fig. 1 for uncropped versions of the images.