Abstract
Obesity is often associated with the risk of chronic inflammation and other metabolic diseases, such as diabetes, cardiovascular disease, and cancer. The composition and activity of the gut microbiota play an important role in this process, affecting a range of physiological processes, such as nutrient absorption and energy metabolism. The active gut microbiota can produce a large number of physiologically active substances during the process of intestinal metabolism and reproduction, including short‐chain/long‐chain fatty acids, secondary bile acids, and tryptophan metabolites with beneficial effects on metabolism, as well as negative metabolites, including trimethylamine N‐oxide, delta‐valerobetaine, and imidazole propionate. How gut microbiota specifically affect and participate in metabolic and immune activities, especially the metabolites directly produced by gut microbiota, has attracted extensive attention. So far, some animal and human studies have shown that gut microbiota metabolites are correlated with host obesity, energy metabolism, and inflammation. Some pathways and mechanisms are slowly being discovered. Here, we will focus on the important metabolites of gut microbiota (beneficial and negative), and review their roles and mechanisms in obesity and related metabolic diseases, hoping to provide a new perspective for the treatment and remission of obesity and other metabolic diseases from the perspective of metabolites.
Keywords: gut microbiota, metabolic disease, metabolites, obesity
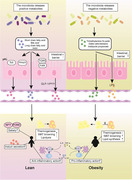
The beneficial and negative metabolites produced by gut microbiota. The gut microbiota produces all kinds of beneficial or negative metabolites and directly affects the intestinal barrier and gut hormone (GLP‐1 and PYY) release. After entering circulation, those metabolites regulate satiety, insulin release, adipose tissue function, and systemic immunity, thus influencing obesity and energy metabolism.
1. INTRODUCTION
Obesity is a major global health challenge, and its health risks have attracted wide attention. Obesity is not only manifested in changes in appearance but is also associated with disrupted lipid and glucose metabolism, chronic inflammation, and an increased risk of multiple metabolic diseases, such as diabetes, cardiovascular disease, and cancer. Although many factors contribute to the development of obesity/metabolic diseases, various lines of evidence link these diseases to changes in the composition and activity of the gut microbiome. 1 The gut microbiota includes up to 100 trillion symbiotic microbes that live in the gut, relying on food scraps, intestinal mucus, and more. 2 The normal gut microbiome of the human body is mainly composed of Firmicutes, Bacteroides, Proteus, Actinomycetes, and Fusobacteria, in which Firmicutes and Bacteroides dominate. Some of the main functions of gut microbiota include: (1) resistance to pathogen infection through direct competition for nutrients and attachment sites; (2) affecting intestinal barrier permeability; (3) regulating intestinal immunity; (4) obtaining energy from indigestible diets; and (5) secreting metabolites with different physiological activities. Therefore, changes in the structure and metabolism of gut microbiota affect the host in many ways, including energy absorption, metabolism, and even immunity.
In the context of obesity, the potential of gut microbiota metabolites to contribute to energy absorption and metabolism is interesting. Obesity may be associated with two dominant phyla: Firmicutes and Bacteroidetes. Turnbaugh et al. 3 found that obese mice had a higher relative abundance of Firmicutes in their microbiota and an enhanced ability of gut microbiota to derive energy from diet, the trait that fecal microbiome transplantation (FMT) results suggested could be transferred. Similarly, the gut microbiota of obese people has this signature. 4 Recent studies have linked obesity to a variety of specific bacteria, such as Akkermansia muciniphila, Bifidobacteria, and Lactobacillus. Several independent trials have shown that supplementation with Akkermansia significantly improves metabolism, improves insulin sensitivity, and reduces plasma triglycerides. 5 , 6 Similarly, Lactobacillus and Bifidobacteria also play an important role in the balance of human intestinal microecology. For example, Bifidobacteria was used to treat obese youth, and insulin sensitivity was significantly improved. 7 How the gut microbiota specifically affects and participates in metabolic and immune activities, especially the metabolites directly produced by the gut microbiota, has attracted extensive attention.
In fact, active gut microbiota can secrete a mass of physiological active substances, including short‐chain fatty acids (SCFAs) and secondary bile acids (BAs), in the process of intestinal metabolism and reproduction. These metabolites can enter the whole body blood circulation through the intestinal tract and directly or indirectly affect human metabolism and immune processes. A metagenomic association study and serum metabonomic analysis found that the abundance of Bacteroides thetaiotaomicron in obese individuals decreased significantly and was negatively correlated with serum glutamate concentration. Intragastric administration reduced plasma glutamate concentrations and reduced diet‐induced weight gain and obesity in mice. 8 Another research has shown that people with type 2 diabetes (T2D) were characterized by dysbiosis of gut microbiota, a decrease in the abundance of butyrate‐producing bacteria and an increase in various opportunistic pathogens. 9 Selectively increasing the diversity and abundance of SCFA‐producing bacteria increases host glucagon‐like peptide 1 (GLP‐1) production and decreases metabolically harmful compounds. 10 This evidence suggests that metabolites secreted by microbiota in intestinal tract are key factors in host–microbiota interactions.
Considering the influence of gut microbiota on the occurrence, development, and consequences of obesity and other disease, understanding gut microbiota and its metabolites is helpful to the treatment of obesity. Several studies have shown that gut microbiota metabolites are associated with a variety of host metabolic characteristics, such as obesity, diabetes, and other metabolic diseases. The occurrence and progression of these diseases often involve energy intake, insulin secretion, changes in adipose tissue function, and systemic chronic inflammation. Some previous studies have also proved that gut microbiota metabolites can affect obesity characteristics through these mechanisms. In this review, we will state beneficial and negative metabolites produced by gut microbiota and their mechanisms to affect obesity and related metabolic diseases (Figure 1).
FIGURE 1.
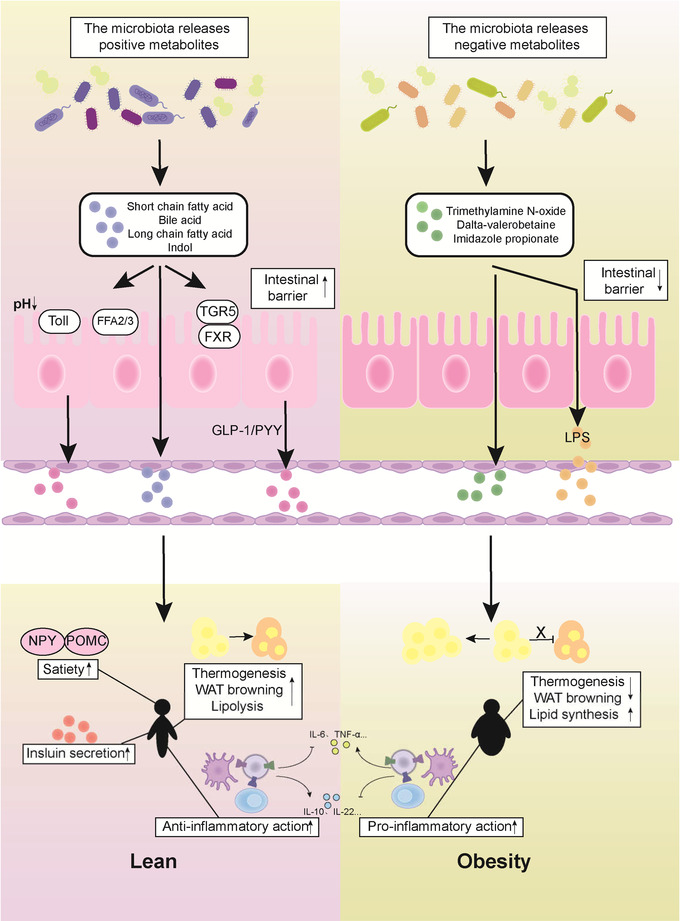
The beneficial and negative metabolites produced by gut microbiota. The gut microbiota produces all kinds of beneficial or negative metabolites and directly affects the intestinal barrier and gut hormone (glucagon‐like peptide 1 [GLP‐1] and tyrosine tyrosine [PYY]) release. After entering circulation, those metabolites regulate satiety, insulin release, adipose tissue function, and systemic immunity, thus influencing obesity and energy metabolism.
2. BENEFICIAL METABOLITES OF GUT MICROBIOTA
Gut microbiota produces a variety of metabolites with physiological effects in the intestinal tract, which are absorbed into the host circulation and can be detected in the blood, brain, and other tissues. SCFAs and secondary BAs, which are beneficial metabolites, are the focus of recent studies. Sufficient human and animal studies have proved that SCFAs and BAs are associated with obesity and metabolic disorders, and they are involved in the processes of nutrient intake, insulin secretion, and immunity to affect the characteristics of obesity in hosts. However, indoles and long‐chain fatty acids (LCFAs) are rarely studied, and most of them only prove their correlation with obesity characteristics, waiting for further research on the mechanism. The intermediate substance of mutual crosstalk between intestinal flora and host. This section introduces four important metabolites of gut microbiota that are thought to be beneficial.
2.1. Short‐chain fatty acids
SCFAs is one of the most studied and common metabolites of gut microbiota, generally including butyrate, propionate, and acetate. It is produced by intestinal bacterial fermentation of undigested food components. After being absorbed by colon cells, these SCFAs are used locally as an energy source for the epithelial cells of the colon mucous membrane or enter the portal vein bloodstream for circulation. Absorption of SCFAs in the gut: diffusion of undissociated forms and active transport of dissociated forms by SCFAs transporters. There are two types of SCFAs transporters: monocarboxylate transporters (MCT1/MCT4) 11 and sodium‐coupled monocarboxylate transporters (SMCT1/SMCT2) that are coupled with a transmembrane H+ gradient. 12 MCT1, SMCT1, and SMCT2 transporters are expressed in the apical membrane of the intestinal epithelium, while MCT1 and MCT4 are expressed in the basolateral region. Transporter expression is regulated by SCFAs and inflammation, and the uptake of SCFAs may be altered in obesity and certain disease states. 13 , 14 Therefore, there may be bidirectional regulation between obesity or other diseases and SCFA levels.
Acetate is the most abundant SCFAs in the colon, accounting for more than half of the total SCFAs detected in feces. 15 There are two main ways to produce acetate: through fermentation or synthesis of acetate through the Wood–Ljungdahl pathway. 16 Propionate is synthesized in three ways: the succinate pathway (Bacteroidetes and some Firmicutes bacteria), acrylate pathway (Veillonellaceae, Coprococcus catus, etc.), and the propanediol pathway (members of the Proteobacteria and Lachnospiraceae families). 17 Two different butyrate production pathways are known: the butyrate kinase pathway and the butyryl‐CoA:acetate CoA‐transferase pathway. 18 In addition, some bacteria do not produce SCFAs themselves, such as Lactobacillus and Bifidobacterium, and their decomposition of dietary fiber provides the necessary substrates (i.e., oligosaccharides, lactic acid, and acetate) for use by other SCFA‐producing bacteria. 19 , 20
Clinical studies found that the total amount of SCFAs in obese subjects was significantly higher than that in lean subjects. 21 The number of SCFA‐producing bacteria and SCFAs decreased in fecal samples from metabolically abnormal mice, as well as from obese and diabetic patients. The composition of the gut microbiota in mice treated with alternate‐day fasting changed, and the levels of acetate and lactic acid in serum increased. 22 An increase in total SCFAs level may indicate an increased ability of obese individuals to extract calories from otherwise indigestible foods. An increase in acetate levels could mean it is metabolically beneficial or that the microbes that produce acetic acid are more likely to increase in this way.
After metformin treatment, T2D patients showed increased fecal potential for butyrate production. 23 Another study further demonstrated an increase in serum acetate and butyrate after 6 months of metformin use. 24 Treatment with a variety of probiotics (Lactobacillus plantarum, Lactobacillus bulgaricus, Lactobacillus gasseri, etc.) in combination with metformin showed increased plasma butyrate concentration and enrichment of microbial butyrate production pathways. 25 An increase in serum acetate was associated with a decrease in fasting insulin, 24 , 26 and increased butyrate production in the gut was associated with improved insulin sensitivity. 27 , 28 Moreover, supplementation with SCFAs can improve metabolism by increasing energy expenditure, glucose tolerance, and homeostasis in diabetic and obese animals. 10 , 29 , 30 In humans, SCFAs stimulate the production of GLP‐1 and tyrosine tyrosine (PYY), leading to weight loss. 31 In normal male mice, studies of Lepob/ob and PPAR‐γLox/Lox mice demonstrated that butyrate can improve metabolism and reduce obesity by promoting GLP‐1 and PYY release and fatty acid oxidation and enhancing mitochondrial function. 32 , 33 , 34
However, another study in pregnant female Sprague–Dawley (SD) rats found that butyrate was associated with increased intramural fat deposition and insulin resistance in offspring. 35 Although the effect of butyrate on metabolism is still controversial, SCFAs represented by butyrate are thought to improve obesity and metabolism in general.
2.2. Bile acids
BAs are produced in the liver as a water‐soluble, cholesterol‐derived amphiphilic molecule of saturated hydroxylated C‐24 sterols. Rate‐limiting enzymes are CYP7A1 (the classical pathway) and sterol‐27‐hydroxylase (CYP27A1) (the alternative pathway). 36 The main BAs produced in humans are cholic acid (CA) and chenodeoxycholic acid (CDCA), while the main BAs produced in rodents are CA and muricholic acid (MCA), especially β‐MCA, at the primary level. After BAs are combined with taurine (mostly in mice) or glycine (mostly in humans), the primary BAs are secreted from the liver into bile, and food is ingested into the duodenum. In distal ileum intestinal cells, 95% of BAs are reabsorbed by the apical sodium‐dependent bile acid transporter (ASBT/SLC10A2) through enterohepatic circulation (EHC). Transport back to the liver, another 5% of BAs are eliminated through feces. 37
The gut microbiota converts primary BAs into secondary BAs through the following main reactions. 38 Bile salt hydrolases (BSHs) cleave the amide bond between the glycine and taurine moieties conjugated to the steroid nucleus of bile salts to liberate BAs, which is the crucial first step for further BAs alterations by microbes within the intestinal environment. BSHs are represented in bacteria, including L actobacillus, Bifidobacterium, Enterococcus, Clostridium spp, est. 39 Conjugated BAs in the gut are known to be toxic to bacteria, especially at low pH, and affect bacterial growth in different areas of the gastrointestinal tract (GIT). As a result, the presence of BSHs can exert a protective effect on certain bacterial species. 40 After that, bile salts were liberated by BSHs, and further metabolism by microbiota began. These BA modifications are performed mainly by anaerobes in the lower intestine and include reamidation, oxidation–reduction reactions, and esterification and desulfation reactions. 41 Bacteria with 7α‐dehydroxylation activity form deoxycholic acid (DCA) and lithocholic acid (LCA) in the human intestine by CA and CDCA, respectively, while αMCA and βMCA form murideoxycholic acid (MDCA) in the mouse intestine. This process occurs mainly in Clostridium firmicutes or Eubacters. 42 Bacteria can also undergo oxidation, epimerization, and dihydroxylation through hydroxysteroid dehydrogenases (HSDHs). 39 , 43 Secondary BAs showed strong antibacterial activity and cytotoxicity.
Many studies have documented changes in plasma BAs levels in metabolic diseases such as obesity and diabetes. 44 , 45 Because BAs are a key ligand for the Takeda G‐protein‐coupled receptor 5 (TGR5) and the farnesoid X receptor (FXR), which regulate glucose and lipid metabolism, signaling affects many different metabolic processes in the host, TGR5 is activated primarily by the secondary BAs LCA and DCA, while the main ligand for FXR is CDCA, followed by CA, DCA, and LCA. BAs change significantly in patients with metabolic diseases such as obesity, and insulin resistance is associated with an increase in total. 46 The level of ursodeoxycholic acid (UDCA) in EHC of high‐fat diet (HFD) rats decreased, while the level of DCA increased, accompanied by an increase in Coprococcus, Lactococcus, Blautia, and Ruminococcus. 47 In obesity‐resistant mice, secondary BAs, including UDCA, CDCA, and LCA, were reduced and were associated with changes in the gut microbiota, such as increases in Akk. muciniphila and uc_Atopobiaceae. The correlation found that C lostridium scindens and Clostridium hylemonae were positively correlated with UDCA, LCA, and DCA. 48 In addition, feeding UDCA and LCA significantly improved fasting glucose, insulin resistance, and obesity in HFD mice. 48 , 49 An increase in serum BAs means that fewer BAs are excreted in the stool. Some studies proved this: patients undergoing bariatric surgery with Roux‐en‐Y gastric bypass (RYGB) had reduced fecal levels of primary and secondary BAs, such as CDCA, CA, LCA, DCA, and UDCA, possibly due to BSH activity from intestinal bacteria, such as Blautia and Veillonella. Its absorption and recycling are increased through the enterohepatic pathway. 50 , 51 In addition, the CA/CDCA ratio was increased in the early feces of RYGB recipients. 50 Another study showed an increased circulating CA/CDCA ratio in pregnant women with intrahepatic cholestasis, which is associated with increased fasting triglycerides and elevated blood sugar. 52 This indicates that the circulating CA/CDCA ratio is closely related to blood glucose and metabolism.
Some probiotics have the ability to regulate BAs metabolism. In vitro evidence confirmed that Parabacteroides distasonis can convert primary BAs to secondary BAs (LCA and UDCA), activate FXR‐fibroblast growth factor 15 (FGF15), and reduce weight gain, hyperglycemia, and hepatic steatosis in ob/ob and HFD mice. 49 Primary BAs (CA and CDCA) decreased and secondary BAs (DCA and LCA) increased after Lactobacillus acidophilus was fed to mice with high cholesterol. 53 After feeding A. muciniphila and quercetin, total plasma BAs increased, and secondary BAs increased overall, especially ωMCA and UDCA. The combination therapy reduced the levels of fasting blood glucose and plasma insulin and prevented obesity and inflammation. 54 In addition, the CA/(glychocholic acid (GCA) + taurocholic acid (TCA) ratio was positively correlated with A. muciniphila, which may be related to BSHs. 54 Lactis 420™ (B420) was administered alone and in combination with prebiotics. The results showed changes in microbial and plasma BAs. Treatment increased Christensenellaceae spp., Bifidobacterium S24‐7, Barnesiellaceae spp., A. muciniphila, Streptococcus, and Lactobacillus and was accompanied by reductions in plasma BAs, including glycocholic acid, glycoursodeoxycholic acid, taurohyodeoxycholic acid, and tauroursodeoxycholic acid (TUDCA). 55
2.3. Long‐chain fatty acids
LCFAs are saturated and/or unsaturated fatty acids containing 14–20 carbons and play an important role in regulating energy metabolism. Recent studies have shown that the gut microbiota Enterococcus faecalis can drive the production of LCFAs (specifically myristoleic acid) through ACOT genes. HFD mice supplemented with E. faecalis showed weight loss, increased energy metabolism, and brown adipose tissue (BAT) activation. 56 In another study, Escherichia coli Nissle 1917 (EcN) and Holdemanella biformis were shown to produce 3‐hydroxy‐octadecenoic acid (C18‐3OH), which is involved in the in vivo anti‐inflammatory process and excites peroxisome proliferator‐activated receptor γ (PPAR‐γ). 57 At present, there are few studies on LCFAs and gut microbiota, and LCFAs are mainly ingested from food. 58 Therefore, the role of LCFAs derived from the gut microbiota in obesity and metabolic diseases needs further research.
2.4. Metabolites of tryptophan
Tryptophan is an essential amino acid found in milk, cheese, fish, and other foods. 59 Tryptophan is a precursor to the synthesis of many important biologically active molecules, such as serotonin, melatonin, niacinamide, and vitamin B3, among many other important substances. Tryptophan is metabolized through three pathways: the serotonin, kynurenine, and indole pathways.
Most dietary tryptophan is metabolized through the kynurenine pathway, through the rate‐binding enzyme indoleamine 2,3‐dioxygenase 1 (IDO1) in immune cells and intestinal epithelial cells, or to NAD+ in the liver by tryptophan 2,3‐dioxygenase (TDO). Part of tryptophan is synthesized into 5‐hydroxytryptamine (5‐HT) in intestinal chromaffin cells. In addition, approximately 5% of tryptophan is metabolized in the intestinal tract by the bacterial tryptophanase of the gut microbiota into a series of indole metabolites, such as indole‐3‐aldehyde (IAld), indole‐3‐acid‐acetic (IAA), indole‐3‐propionic acid (IPA), and indole‐3‐lactic acid (ILA). 60
Clinical studies found that the levels of IAA, ILA, and IPA were significantly reduced in obese subjects. 61 Among them, IPA and IAA are the most important indole metabolites. IPA reduces the risk of T2D and has been shown to be associated with gut microbiota diversity. 62 Similarly, mice fed with HFD had lower levels of IPA and IAA in their serum. 63 , 64 A higher IPA concentration was negatively correlated with the incidence of diabetes but positively correlated with higher dietary fiber intake, suggesting that gut microbiota may play an important role. 65 Indole metabolites, including IAA, ILA, and IPA, were negatively correlated with 5‐HT and kynurenine/tryptophan levels in obese people. This suggests that the metabolism of tryptophan through the gut microbiota pathway is decreased, while the kynurenine and serotonin pathways are increased under the influence of obesity. 61
Tryptophan catabolism bacteria that produce indole derivatives include anaerobes, Bacteroides, Clostridium, Bifidobacterium, and Lactobacillus. Tryptophan and indole active transporters have been demonstrated in E. coli, Lactobacillus reuteri, and Lactobacillus johnsonii catalyze tryptophan catabolism to IAld by the enzyme aromatic amino acid aminotransferase. 66 However, because the indole pathway of tryptophan requires a series of enzymes and different bacteria have different metabolic enzymes, it is difficult to accurately describe the enzymatic pathway of bacterial indole metabolite production. 67 Probiotics such as Akkermansia, Lactobacillus, and Bifidobacteria can affect tryptophan metabolism. 68 In a recent study, A. muciniphila, either live or pasteurized, reduced elevated serum levels of tyrosine, phenylalanine, and tryptophan metabolites in diabetic patients. 69 The intestinal flora of mice fed with a HFD changed, the abundance of Bacteroidetes and Akkermansia decreased, and the serum IAA level decreased. 63 However, how Akkermansia reduces the tryptophan metabolite level remains to be further explored. Another study of the oral administration of Bifidobacterium longeum BL21 in male C57BL/6J mice suggests that probiotics may affect tryptophan metabolism by balancing the gut microbiota (Figure 2). 70 , 71
FIGURE 2.
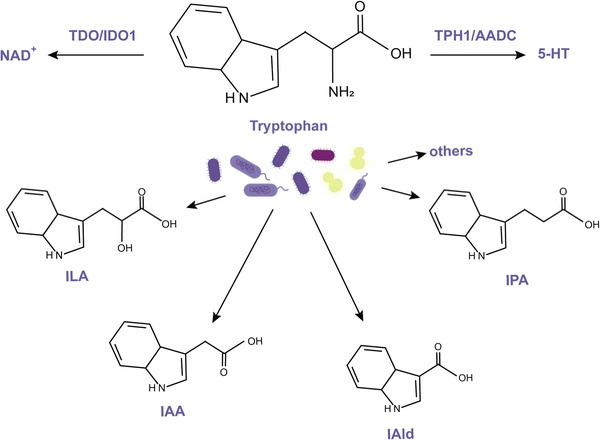
Tryptophan metabolism. Most dietary tryptophan is metabolized to NAD+ in the liver. Part of tryptophan is synthesized into 5‐hydroxytryptamine (5‐HT). Approximately 5% of tryptophan is metabolized in the intestinal tract by the bacterial tryptophanase of the gut microbiota into a series of indole metabolites, such as indole‐3‐aldehyde (IAld), indole‐3‐acid‐acetic (IAA), indole‐3‐propionic acid (IPA), and indole‐3‐lactic acid (ILA).
3. NEGATIVE METABOLITES OF GUT MICROBIOTA
This section introduces three negative metabolites: trimethylamine N‐oxide (TMAO), delta‐valerobetaine (VB), and imidazole propionate (ImP). TMAO is an important metabolite among them, which has been proved to be related to host metabolic disorders and obesity, and it has been verified that it can indeed play a role in the development of obesity. At present, there are few studies on these negative metabolites, which are mainly related to systemic chronic inflammation and adipose tissue function changes, and other mechanisms need to be further explored. But what is debatable is whether these metabolites are markers of impaired metabolism, the result of impaired metabolism, or both?
3.1. Trimethylamine N‐oxide
TMAO is a trimethylamine (TMA) metabolized from nutrients in food (such as phosphatidylcholine, choline, etc.) by intestinal microbial enzymes (such as CutC/D and CntA/B), which are then further metabolized by the host enzyme flavin‐containing monooxygenase 3 (FMO3) in the liver. 72 Choline catabolism increases the level of circulating TMO.
In several separate human and animal studies, TMAO has been shown to increase obesity, T2D, and insulin resistance. 73 , 74 , 75 , 76 , 77 It has been found that a HFD impairing mitochondrial function in the colonic epithelium enhances the enteric bioavailability of oxygen and nitrate, thereby enhancing the respiration‐dependent choline catabolism of E. coli. 78 After weight loss and RYGB, plasma TMAO levels decrease, along with obesity and insulin resistance. 79 , 80 Dietary interventions in obese and glucose‐intolerant patients can reduce TMAO levels and regulate postprandial glucose levels. 81
Currently, TMA‐producing bacteria are reported as obligate anaerobic Clostridia (phylum Firmicutes) and facultatively anaerobic Enterobacteriaceae (phylum Proteobacteria). 82 , 83 A new microbial enzyme, CntA/B, has been found to convert carnitine to TMA, a gene present in only a few species, including E. coli, Klebsiella spp., and Citrobacter spp. 84 Treatment of mice with the inhibitor iodimethylcholine to inhibit the TMA lyase CutC in gut microbiota and thereby reduce plasma TMAO levels showed effective inhibition of diet‐induced obesity and altered the expression of lipid metabolism genes in white adipose tissue (WAT). 85 In addition, the choline analog 3,3‐dimethyl‐1‐butanol and the carnitine analog mildronate have also been shown to reduce TMAO levels. 86 Moreover, TMAO inhibited hepatic BAs synthesis. 87
3.2. Delta‐valerobetaine
VB is a microbial source of diet‐dependent obesogen. It was found to reduce carnitine levels and inhibit mitochondrial fatty acid oxidation in mice. 88 Because carnitine regulates glucose and lipid metabolism, 89 VB is the substrate for carnitine transporter (OCTN2). Therefore, VB may reduce the efficiency of carnitine reuptake, that is, increase the elimination rate of urinary carnitine.
To determine whether the gut microbiota produces VB, the cecal contents of both normal and germ‐free mice were cultured in vitro. The results showed that only normal cecal contents produced VB. It was found that E. coli (K12) and Salmonella typhimurium produced VB in isolated cultures. Furthermore, VB is one of the precursors of TMA. 88 This indicated that VB may combine with TMA/TMAO to enhance metabolic disorders and cause obesity.
3.3. Imidazole propionate
ImP is a metabolite produced by gut microbiota using histidine and is found at higher levels in the blood of T2D and pre‐T2D patients. This means that ImP is associated with impaired glucose metabolism, not with diabetic status. 90 Analysis of gut microbiota in T2D patients showed that ImP levels were associated with a low abundance of microbial diversity and Bacteroidetes. Another study also demonstrated that ImP can predict the α diversity of human gut microbiota. 91 ImP increases systemic inflammation and is negatively associated with circulating mucosal‐associated invariant T (MAIT). An unhealthy diet (not histidine intake) alters ImP levels, so it is possible that diet alters gut flora and thus ImP levels. In other words, ImP levels may be a symptom of metabolic impairment and contribute to disease development.
4. MECHANISMS AFFECTING OBESITY/METABOLIC DISEASE
Obesity is often associated with an imbalance in energy intake and expenditure, insulin resistance, and chronic inflammation. As previously stated, intestinal flora regulates a variety of host physiological functions, such as energy absorption, intestinal barrier permeability, secondary BA synthesis, and intestinal hormone release. After its metabolites enter the circulation, they can affect appetite through the blood–brain barrier, and affect adipose tissue synthesis, decomposition, thermogenesis, and browning. At the same time, the influence of intestinal flora on intestinal permeability is also associated with systemic chronic inflammation, which is a further factor leading to insulin resistance. 92 Several mechanisms of intestinal microbiota metabolites affecting obesity have been proposed.
4.1. Affect host satiety and energy intake
An important factor causing obesity is that the body absorbs more energy than it consumes, and the excess energy is stored in the form of fat. Food intake and digestion are controlled by the interaction between the GIT and the brain. Gut hormone (GLP‐1, PYY, etc.) and neural signals from the GIT are key participants in this bidirectional signaling pathway.
Gut hormones secreted by L cells, such as GLP‐1 and PYY, are involved in the regulation of food intake, intestinal motility, and insulin secretion. SCFAs, BAs, and indoles produced by gut microbiota can affect the release of gut hormones through different pathways. Peripheral BAs activate TGR5 in intestinal L cells to stimulate the release of GLP‐1, thereby improving glucose tolerance and obesity. 93 FXR is another important target of BAs. Treatment with the BA‐sequestering resin sevelamer increased GLP‐1 secretion 94 and improved blood glucose in ob/ob mice in an FXR‐dependent manner, confirming that the FXR/GLP‐1 pathway is a novel mechanism by which BAs regulate glucose metabolism. 95 SCFAs stimulate the release of GLP‐1 and PYY from intestinal L cells via the G protein‐coupled free fatty acid receptors FFAR2 (GPR43) and FFAR3 (GPR41). 96 , 97 In addition, butyrate can also increase PYY expression by upregulating Toll‐like receptors. 98 PYY affects appetite and satiety by inhibiting neuropeptide Y (NPY) and activating proopiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus (ARC) or by delaying gastric emptying. GLP‐1 indoles have been shown to stimulate GLP‐1 production in intestinal endocrine L cells. 99 In vitro experiments have shown that indoles inhibit voltage‐gated K+ channels, increase the time width of action potential stimulated by L cells, and lead to enhanced Ca2+ entry, which dramatically stimulates GLP‐1 secretion. 100
In addition to directly affecting gut hormone production, gut microbiota metabolites can also suppress appetite through the central nervous system. TGR5 was found to be expressed in neurons in the hypothalamus. BAs are also found in the brain, where levels correlate with levels in the circulation. Thus, a recent study revealed a long‐term BA‐dependent hypothalamic mechanism that contributes to weight regulation in obesity. BAs have been shown to improve diet‐related obesity through TGR5 signal transduction in the hypothalamus, including the regulation of food intake and energy expenditure. 101 Similarly, colonic acetate has been shown to cross the blood–brain barrier to reach the hypothalamus, where it increases the glial circulation of glutamate‐glutamine and gamma‐aminobutyric acid (GABA), increasing lactate levels and thereby suppressing appetite. 102 In addition, oral butyrate inhibited the activity of appetite‐stimulating neurons expressing NPY in the hypothalamus, decreased the activity of neurons in the solitarytract nucleus and dorsal vagus nerve complex in the brain stem, and ultimately indirectly interfered with the appetite and feeding behavior of the host (Figure 3). 103
FIGURE 3.
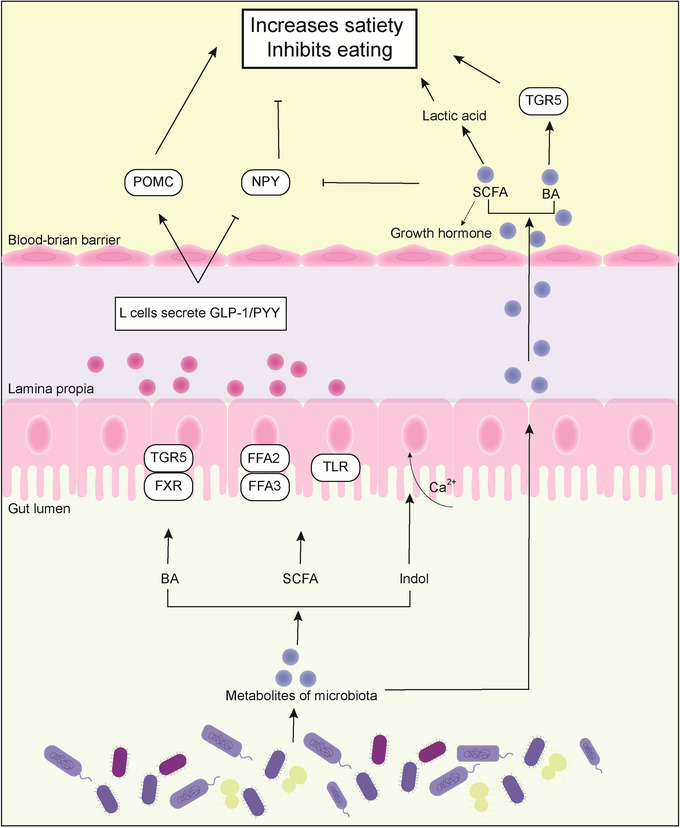
Metabolites enhance satiety and inhibit eating. Through bile acid (BA) receptors (Takeda G‐protein‐coupled receptor 5 [TGR5] and farnesoid X receptor [FXR]), short‐chain fatty acid (SCFA) receptors (GPR41/43), Toll‐like receptor (TLR) of intestinal cells, and enhanced Ca2+ entry, L cells can promote the release of intestinal hormones (glucagon‐like peptide 1 [GLP‐1]/tyrosine tyrosine [PYY]), activate hypothalamus proopiomelanocortin (POMC) and inhibit neuropeptide Y (NPY) to improve satiety. Meanwhile, BAs and SCFAs can cross the blood–brain barrier, and BAs enhance satiety through TGR5 receptors in the hypothalamus. SCFAs increase lactic acid and inhibit NPY. Otherwise, SCFAs promote the level of growth hormone, which can control energy homeostasis by stimulating lipolysis and protein retention.
4.2. Affect islet β cells and insulin secretion
Islet β cells play an important role in the development of obesity and diabetes by producing and storing large amounts of insulin, which is secreted precisely into the circulation when stimulated by glucose. SCFAs and BAs promote glucose‐stimulated insulin secretion (GSIS) through several pathways.
First, SCFAs may contribute to the β cell response to insulin resistance. The SCFA receptor FFA2/3 is expressed in islet β cells. FFA2/3 levels were upregulated in the islets of HFD mice, although there is some controversy. 104 FFAR2−/− mice fed with HFD showed impaired glucose tolerance and reduced insulin secretion. 105 One study demonstrated that FFAR2−/− mice were obese on a normal diet, whereas mice that overexpressed FFAR2 maintained a lean phenotype even when fed with HFD. Moreover, both types of mice reared under sterile conditions or after antibiotic treatment had normal phenotypes, suggesting an important role for the gut microbiota. 106 The mechanism is that SCFAs are activated in combination with FFA2/3. FFA2 regulates GSIS by Gαq/11 (primary) and Gαi/o, whereas FFA3 inhibits GSIS by Gα i/o. In β cells, increased Ca2+ triggers insulin secretion. 107
Second, islet β cells also express the BA receptors TGR5 and FXR. Stimulation of the islets of male mice with TUDCA was found to increase GSIS in a dose‐dependent manner. 108 In vitro experiments have shown that selective TGR5 ligands lead to increased insulin secretion in a manner dependent on G s/cAMP/Ca2+.93 This mechanism also relies on protein kinase A (PKA), since inhibition of PKA reduces TUDCA‐promoted GSIS secretion. 108 , 109 In addition, BAs can increase Ca2+ and promote insulin secretion through FXR expressed by islet β cells. 110
In addition to acting directly on islet β cells, as mentioned above, the metabolites of gut microbiota BAs, SCFAs, and indole can also promote GSIS indirectly by acting on islet β cells with GLP‐1. 111 GLP‐1 has the following effects on islets: (1) it stimulates insulin secretion; (2) it increases glucose metabolism by promoting insulin synthesis; and (3) it stimulates β cell proliferation and inhibits apoptosis (Figure 4). 112
FIGURE 4.
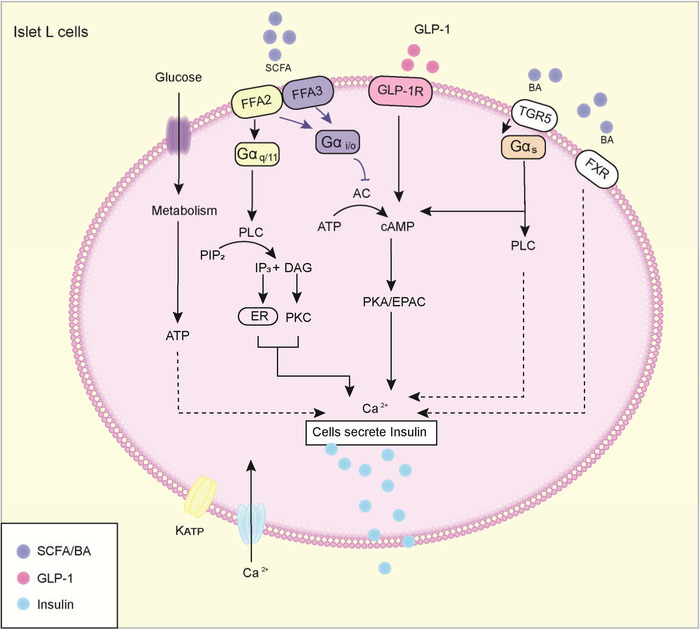
Metabolites affect islet β cells and regulate insulin secretion. When glucose internalized in islet β cells is metabolized, it results in elevation of the adenosine triphosphate (ATP):adenosine diphosphate (ADP) ratio, closure of ATP‐sensitive potassium (KATP) channels, and opening of voltage‐dependent calcium channels (Ca2+ channels), causing secretion of insulin. First, short‐chain fatty acids (SCFAs) can improve or decrease glucose‐stimulated insulin secretion (GSIS). After Free fatty acid 2 (FFA2) activation, the Gαq/11 subunit activates phospholipase C (PLC), which hydrolyzes phosphatidylinositol 4,5‐bisphosphate (PIP2) to diacylglycerol (DAG) and inositol triphosphate (IP3), activating protein kinase C (PKC) and releasing calcium ions from endoplasmic reticulum (ER) storage, respectively, which can promote insulin release. FFA2 and FFA3 can also bind to Gα I/O subunits and inhibit adenylate cyclase (AC), thereby reducing the concentration of cyclic AMP (cAMP) and inhibiting protein kinase A (PKA) and exchange protein directly activated by cAMP (EPAC)‐mediated insulin release. Similarly, bile acids (BAs) activate the Takeda G‐protein‐coupled receptor 5 (TGR5) receptor to bind Gα s and promote insulin release through the PLC and cAMP pathways. BAs can also bind to farnesoid X receptor (FXR) receptors to promote Ca2+ release. Meanwhile, metabolites (SCFAs, BAs, and indole) promote the release of glucagon‐like peptide 1 (GLP‐1) by intestinal L cells, and its binding with GLP‐1R also promotes the release of insulin.
4.3. Affect adipose tissue function
Due to adaptive thermogenesis, BAT has become a hot topic in obesity and related metabolic diseases. BAT can metabolize energy substrates to produce heat but does not release chemical energy from adenosine triphosphate (ATP) decomposition. This process is driven by uncoupling protein 1 (UCP‐1). Therefore, increased BAT quality in obese patients may improve metabolism. One possible way to increase the presence of UCP‐1 in adipose tissue is to convert white (pre) adipose cells to brown‐like adipose cells, leading to fat browning. 113 Both human and mouse studies have proven that BA can induce the expression of UCP‐1 in BAT, increase energy conversion and reduce obesity. 114 , 115 A human study showed that BAT activity was negatively correlated with the 12α‐OH/non12α‐OH BA ratio, which may represent the key enzyme CYP8B1 as a target of BAT. 116 CDCA, LCA, and other TGR5 agonists increase UCP‐1 expression in brown fat cells, suggesting that TGR5 plays an important role in BA‐mediated fat browning. 115 Activation of TGR5 in BAT increases thermogenesis by converting inactive thyroxine (T4) to active thyroid hormone (T3). 117 In addition, UDCA was shown to induce a significant elevation of SIRT‐1‐PGC‐1α in adipose tissue. 118 Myristoleic acid, a type of LCFA from gut microbiota E. faecalis, also reduces obesity by activating BAT and forming beige fat. 56 It has been shown that another LCFA, eicosapentaenoic acid, induces thermogenesis of BAT cells through upregulation of FFAR4‐dependent miR‐30b and miR‐378. 119 In addition, LCFAs also activate UCP‐1 in fat. Importantly, a recent study showed that LCFAs are essential for UCP‐1 decoupling. 120 However, the role of intestinal microbiome‐derived LCFAs in obesity and metabolic diseases needs further research. In contrast, TMAO may decrease PGC‐1α expression. When the TMAO‐producing enzyme FMO3 was knocked down, the expression of browning and thermogenic genes such as UCP‐1 was increased. The dietary supply of TMAO reversed the increase in PGC‐1α in WAT driven by knockdown of FMO3. 121 In addition, TMAO and TMA may be related to insulin signaling in adipocytes, with hormone‐like signaling. 122
The imbalance of lipid decomposition and synthesis in adipose tissue is one of the factors leading to obesity and related diseases. In mouse 3T3‐L1 adipocytes, butyrate, and propionate have been shown to promote intracellular lipolysis by activating FFAR2/3. 123 Butyrate supplements promote lipid oxidation by downregulating the expression and activity of PPAR‐γ. 34 In addition, SCFAs also affect extracellular fat breakdown mediated by lipoprotein lipase (LPL). 124 In vitro treatment of porcine preadipocytes with acetate, butyrate and propionate enhanced adipocyte differentiation, suggesting that SCFAs have lipogenic properties. 125 This may be related to GPR43/FFAR2. In contrast, VB, a diet‐dependent obesogen, has been shown to promote tissue fat accumulation and reduce fatty acid utilization by reducing carnitine levels. 88 Growth hormone has previously been reported to play an important role in the control of energy homeostasis by stimulating lipolysis and protein retention. 126 SCFAs can also have positive effects by regulating growth hormone secretion and metabolism. 127 Butyrate can stimulate growth hormone synthesis and promote growth hormone secretion induced by basal hormones and growth hormone‐releasing hormones. The results show that butyrate can improve lipolysis and oxidative metabolism (Figure 5). 128
FIGURE 5.
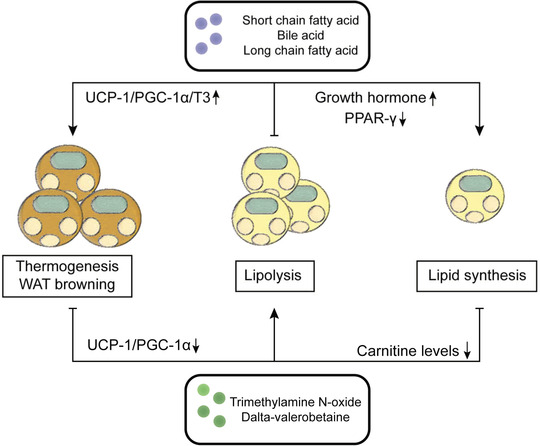
Metabolites affect adipose tissue function. Under the action of beneficial metabolites (short‐chain fatty acids [SCFAs], bile acids [BAs], long‐chain fatty acids [LCFAs]), adipose tissue promotes adipose thermogenesis and browning through uncoupling protein 1 (UCP‐1)/peroxisome proliferator‐activated receptor gamma‐coactivator 1α (PGC‐1α)/T3, promotes adipose decomposition through growth hormones produced by metabolites in the brain to balance energy metabolism in adipose tissue. In contrast, metabolites (trimethylamine N‐oxide [TMAO] and delta‐valerobetaine [VB]) inhibit browning and thermogenesis of adipose tissue, reduce lipid decomposition by reducing carnitine levels, and promote infiltration of immune cells and proinflammatory cytokines.
4.4. Affect chronic systemic inflammation
Systemic inflammation, characterized by an increase in the number of immune cells and tissue infiltration, is closely associated with insulin resistance and obesity. 129 This is manifested by impaired intestinal barrier function and infiltration of inflammatory cells (CD4+ T cells, M1 macrophages, natural killer [NK] cells, and innate lymphocytes [ILCs]) into WAT, which promotes a proinflammatory state and leads to the development of the disease.
The establishment of a chronic inflammatory state and an increase in intestinal permeability are associated with an increase in lipopolysaccharide (LPS) in the host circulation. The establishment of chronic inflammatory states and increased intestinal permeability are associated with elevated levels of systemic LPS. This is well demonstrated in animal and human studies of obesity and related disease development. 130 SCFAs, BAs, and indoles can enhance intestinal barrier function, reduce LPS circulation through the intestinal barrier, and reduce inflammation in the body. SCFAs (particularly butyrate) improve epithelial barrier function and intestinal permeability by regulating tight junction protein and mucin expression, and the reduced lumen pH adds an additional layer of protection against pathogen invasion and colonization. 131 Tryptophan metabolism pathway changes in obese people are associated with systemic inflammation. 61 IPA was shown to reduce the increase in high intestinal permeability and LPS in HFD‐fed mice. 71 The mechanism is to improve intestinal permeability and inflammation by upregulating connexin‐coding mRNAs (ZO‐1, Occludin, and Claudin‐5). 64 , 132
These metabolites also have the ability to control the host immune response by regulating the differentiation, recruitment, and activation of neutrophils, dendritic cells (DCs), macrophages, monocytes, and T cells. First, neutrophils are important parts of the innate immune system. They reach the site of inflammation first and recruit other cells, including macrophages, by producing cytokines. 133 SCFAs directly affect neutrophils and regulate the production of inflammatory cytokines. In vitro experiments reported that acetate and butyrate reduced the secretion of tumor necrosis factor‐α (TNF‐α) and nuclear factor κB (NF‐κB) from LPS‐stimulated neutrophils. 134 In vitro supplementation of mice with acetate prevented the accumulation of neutrophils in the colon induced by a fiber‐free diet. 135
Monocytes are large phagocytes which can turn into macrophages and DCs, and play a critical role in adaptive immune responses. SCFAs inhibit the maturation of monocytes, macrophages, and DCs, alter their ability to capture antigens, and reduce their production of proinflammatory cytokines (such as interleukin [IL]‐12 and TNF‐α). Similarly, BAs exert an anti‐inflammatory response through activation of TGR5 in macrophages, increasing intestinal IL‐10 levels 136 and inhibiting the NF‐κB signaling pathway 137 and NLRP3 inflammasome. 138
The number of regulatory T cells (Treg cells), which are important central regulators of antimicrobial immunity and tissue inflammation, is reduced in the visceral adipose tissue of obese individuals. SCFAs can rely on FFA2 to increase the number of Tregs and induce differentiation. 132 , 139 FFAR2 was found to increase the number of Tregs in the body. 140 In addition to its direct promotion of Treg cell differentiation, butyrate enhanced the ability of DCs to promote Treg differentiation. 141 BAs can regulate intestinal RORγ+ Treg cell homeostasis, 142 increase Treg cell differentiation, and directly regulate the TH17/Treg cell balance. 143 Indoles produced by intestinal flora metabolizing tryptophan can regulate miR‐181 in white adipocytes, increase Treg, eosinophils, ILCs and M2 macrophages, and inhibit M1 macrophages and TNF‐α, thereby regulating obesity. 144 Because Indoles activate aromatic hydrocarbon receptors (AHRs), they regulate IL‐22 production by ILCs in the intestinal mucosa. While SCFAs promote IL‐22 production in CD4+ T cells and ILCs through histone deacetylase inhibition and GPR41. 145 , 146 Serum TMAO is thought to promote inflammation by increasing proinflammatory genes such as IL‐6 and chemokine ligands (Figure 6). 147
FIGURE 6.
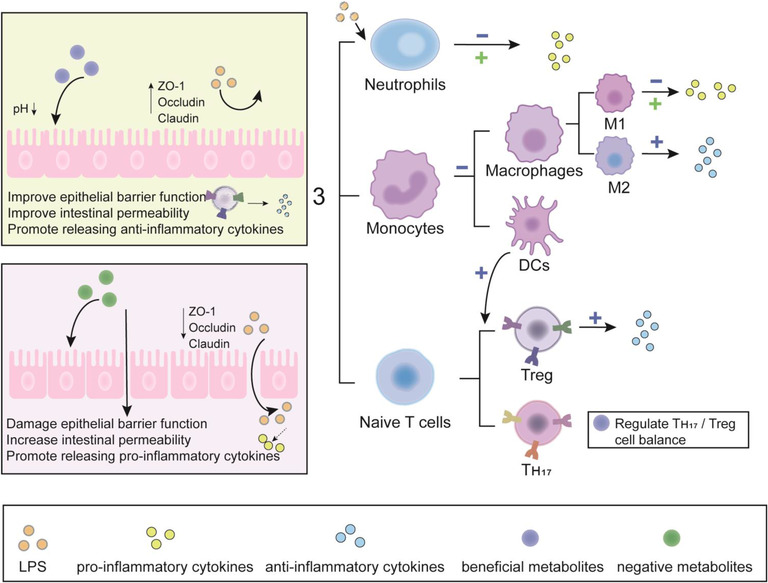
Metabolites affect chronic systemic inflammation. First, the beneficial metabolites in the intestinal tract decrease intestinal pH value and increase the expression of tight junction proteins (ZO‐1, Occludin, and Claudin), improve intestinal barrier and permeability, and reduce lipopolysaccharide (LPS) entry into the body. Metabolites then enter the body, preventing LPS‐stimulated neutrophil production of proinflammatory factors, reducing the maturation of macrophages and dendritic cells (DCs), or promoting the differentiation of M2 anti‐inflammatory cells. In addition, they are able to balance Treg/TH17 cells to regulate immunity while enhancing the ability of DCs to promote regulatory T (Treg) cell differentiation. Negative metabolites increase intestinal permeability and damage the intestinal barrier, and LPS easily enters the body, leading to systemic inflammation.
5. CLINICAL APPLICATION OF GUT MICROBIOTA
Currently, therapeutic drugs and lifestyle interventions are recommended for obese and diabetic patients to alleviate metabolic problems such as obesity and diabetic. However, no matter the lifestyle intervention, medication or RYGB surgery, there are many problems such as poor efficacy, poor drug compliance, and other adverse reactions. 148 , 149 , 150 Many previous animal and human studies have demonstrated the important role of intestinal flora in host energy metabolism. In addition to directly affecting various physiological functions of the host in the intestinal tract, the gut microbiota also indirectly affects the host circulation through metabolites. These studies provide new perspectives and feasible approaches for the application of intestinal flora in the treatment of obesity and related metabolic diseases: probiotics and prebiotics supplementation, FMT, and direct supplementation of the metabolites. More research is needed to confirm that using gut microbiota to treat obesity and related metabolic diseases appears to be a more personalized and safer approach than other therapies. 151 Table 1 shows some examples of clinical applications.
TABLE 1.
List of clinical application of gut microbiota
| Clinical application | Study model | Effects in host related to obesity | Reference |
|---|---|---|---|
| Akkermansia muciniphila | Obese adults |
Insulin sensitivity ↑ Insulinemia and total plasma cholesterol ↓ |
5 |
| SCFAs mixtures | Normoglycemic men |
Fasting fat oxidation, resting energy expenditure ↑ PYY ↑ |
152 |
| Propionate | Overweight adult humans |
PYY, GLP‐1 ↑ Energy intake and weight gain ↓ |
30 |
| BAs | Participants with obesity and diabetes |
Glucose, fructosamine, insulin, LDL, FGF19 ↓ GLP‐1, C‐peptide ↑ |
153 |
| FMT | Obese adolescent | No effect of FMT on weight loss in adolescents with obesity | 154 |
| FMT | Adults with obesity and mild–moderate insulin resistance | No clinically significant metabolic effects during the study | 155 |
| FMT | Obese patients |
Taurocholic acid levels↓ BAs profiles began to resemble No significant changes in mean BMI |
156 |
| FMT | Male with the metabolic syndrome | Not observe metabolic change insulin sensitivity ↑ | 157 |
| FMT | Patients with severe obesity and metabolic syndrome | Improvements in HOMA2‐IR | 158 |
| FMT | Obese subjects with T2D |
Bifidobacterium and Lactobacillus ↑ LDL cholesterol, liver stiffness ↓ |
159 |
| Green‐Mediterranean dieters and FMT | Obese or dyslipidemic participants | Prevents weight gain and improves glucose tolerance | 160 |
Abbreviations: BA, bile acid; BMI, body mass index; FGF, fibroblast growth factor; FMT, fecal microbiome transplantation; GLP‐1, glucagon‐like peptide 1; LDL, low‐density lipoprotein; SCFA, short‐chain fatty acid; T2D, type 2 diabetes.
The most common intervention for the gut microbiota is direct supplementation with specific probiotics and prebiotics. Long‐term consumption of fermented foods such as yogurt and cheese seems to be good for your health. 161 , 162 Well‐known probiotics such as Bifidobacterium and L. plantarum have been added to medicines and foods as bioactive substances to promote human health. 163 , 164 There have been many excellent reviews on this topic. 165 , 166 , 167 It is worth mentioning that A. muciniphila, as a new generation of probiotics, has attracted much attention recently. In rodents, treatment with A. muciniphila reduces manifestations of obesity and improves metabolism, such as glucose tolerance, insulin resistance, steatosis, and intestinal permeability. 168 , 169 In clinical application, A. muciniphila also showed similar effects. After 6 weeks of diet intervention in obese and overweight adults, A. muciniphila was negatively associated with fasting blood glucose, waist‐to‐hip ratio, subcutaneous adipocyte diameter, and plasma triglycerides. 6 More surprisingly, pasteurized A. muciniphila also improved insulin sensitivity, reduced insulinemia, and reduced plasma total cholesterol. 5 This provides a new perspective for providing other probiotic agents.
Subsequently, the lab further demonstrated that A. muciniphila can play a beneficial role by activating PPAR‐α. 170 However, there are not many clinical studies on A. muciniphila, and more independent studies are needed to provide evidence.
In recent years, studies on intestinal microbiota metabolites have mainly focused on their role as the “intermediate bridge” between gut microbiota and host, so there are few studies on the direct supplementation of metabolites in the treatment of obesity and related metabolic diseases in clinical studies. Most are concentrated in SCFAs and BAs. A study in obese and normal male populations using a mixture of SCFAs (including acetate, propionate, and butyrate) for rectal administration showed that SCFAs increased fat oxidation, resting energy expenditure, and PYY secretion in humans. 152 Chambers et al. 30 developed the inulin‐propionate ester for targeted delivery of propionate due to the poor adaptability of oral SCFAs in humans and its rapid absorption in the small intestine. The results showed a significant decrease in body weight, fat, and liver lipids, and prevented deterioration of insulin sensitivity. Another placebo‐controlled, double‐blind, randomized controlled trial using conjugated BAs sodium extract (IC‐CBAs) significantly reduced postprandial glucose, fasting insulin, fasting low‐density lipoprotein (LDL), and increased postprandial GLP‐1 levels. 153 In addition, UDCA is commonly used to reduce symptomatic gallstone disease after RYGB. 171 , 172 , 173 At present, this part of the research field is relatively blank, indicating that the research on new delivery system to alleviate and treat obesity and related diseases is still a little‐traveled road.
FMT is a means of transferring intestinal flora from host to host and has previously been shown to change obesity phenotype and metabolic function in many animal studies. 174 , 175 , 176 , 177 However, in several independent clinical trials, FMT appears to be controversial in improving body weight. Results of a randomized, double‐blind, placebo‐controlled trial of obese adolescents showed that FMT had no effect on weight loss in obese adolescents, despite a reduction in abdominal fat percentage. There was only a temporary improvement in glucose metabolism and insulin sensitivity at 6 weeks. 154 Previous clinical trials have also found that FMT appears to have limited effects on weight and metabolism. 155 , 156 , 157 However, a randomized clinical trial of FMT and fiber supplementation in patients with severe obesity and metabolic syndrome found significant improvements in subjects' insulin sensitivity. 158 FMT and lifestyle intervention increased the abundance of Bifidobacterium and Lactobacillus, and significantly improved the blood lipid and liver hardness of the subjects. 159 Similarly, FMT can be combined with a Mediterranean diet and foods like green tea to maintain weight and stabilize blood sugar. 160 This may be because transplanting gut microbiota alone is difficult to produce a stable effect in the subjects’ intestines, and combined with other methods that facilitate probiotic colonization, FMT could significantly improve obesity and metabolism.
6. DISCUSSION AND CONCLUSION
Previous animal and human studies have highlighted the importance of gut microbiota in regulating host metabolic processes. But there are still some problems. First, are gut microbiota and host bidirectional regulation? Are certain intestinal microbiota metabolites circulating in the host a marker or a consequence of impaired metabolism? ImP, for example, is found at higher levels in the blood of T2D patients and is associated with inflammation, while diet also alters ImP levels by altering gut microbiota. Thus, ImP levels may be a sign of impaired metabolism and, in turn, may contribute to the development of disease. Second, the effect of gut microbiota metabolites on the host is sometimes contradictory in various animal and human studies. Butyrate is generally thought to improve metabolic function. However, one study found that butyrate was associated with increased intramural fat deposition and insulin resistance in the offspring of pregnant female SD rats. 35 Diet, exercise, and a variety of other factors can affect the composition and structure of the gut microbiota. Changes in the gut microbiota, in turn, affect the production of metabolites and subsequently alter a variety of complex physiological activities in the body, as well as the competition/synergy between microbes. Thus, the host–microbe axis can be bidirectional and complex. Furthermore, the mechanism of gut microbiota metabolites has been verified in rodents, but the composition and structure of the human intestinal microbiota are different from those of rodents. Clinical trials of gut microbiota are based on animal studies, but do not necessarily yield similar results. Therefore, it needs to be explored further.
Research in the field of the gut microbiota over the past two decades has shown that human biology is particularly relevant to coexisting microorganisms, most of which live in the digestive tract, where they produce or modify a variety of chemicals that directly or indirectly participate in and influence a variety of physiological functions, including energy metabolism and immune and neurological functions. Although this article cannot cover all aspects of current gut microbiota research, it is certain that substances produced by gut microbiota metabolic activities in the gut will have clinical significance in the future treatment of obesity and related metabolic diseases. We also have some challenges. First, regulating gut microbiota can be used to treat metabolic diseases clinically, because it seems safer and more personalized than other approaches. However, as mentioned in Section 5, the clinical effects of FMT are not satisfactory. It seems that the effects of obesity alleviation and metabolism improvement are not so significant. When combined with other treatments, such as cellulose blends or lifestyle interventions, results are better. Therefore, more stable methods are needed to regulate the gut microbiota. Second, the applications of a new generation of probiotics are what we want. A. muciniphila is a new generation of probiotics. Although many animal experiments have proved its beneficial effects and elaborated its mechanism, few clinical trials have been carried out at present. However pasteurization of A. muciniphila offers a new perspective on new forms of treatment. 5 In addition, the gut microbiota can be used as an adjunct to drug therapy. As clinical trials have demonstrated that probiotics alone can improve glucose homeostasis. 178 Single or multiple probiotics can also assist metformin or other diabetes drugs to treat T2D and improve fasting glucose, insulin resistance and the intestinal barrier. 25 , 179 It can be used as an adjunct to drugs and can be tailored to individual patients. And last, gut microbiota metabolites can also be used as a drug, and new agents can be developed to deliver intestinal microbiota metabolites for better absorption or targeting.
Finally, the gut microbiota is far more complex than previously known. The gut microbiota goes far beyond bacteria, including fungi, phages, and eukaryotic viruses. They compete and collaborate with each other. Moreover, it is important that the large number of active substances produced by gut microbiota affect host physiology and pathology, providing new ideas for the prevention or treatment of common metabolic disorders.
AUTHOR CONTRIBUTIONS
K.L. conceived the concept of the review article and wrote the sections. L.Z. screened and sorted out the references. L.Y. supervised the writing and final revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
The authors declare they have no conflicts of interest.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENTS
All the figures are made by Adobe Illustrator 2020. This work was supported by grants from the 1.3.5 project for disciplines of excellence (No. ZYGD18007), West China Hospital, Sichuan University.
Lin K, Zhu L, Yang L. Gut and obesity/metabolic disease: Focus on microbiota metabolites. MedComm. 2022;3:e171. 10.1002/mco2.171
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Sonnenburg JL, Bäckhed F. Diet‐microbiota interactions as moderators of human metabolism. Nature. 2016;535(7610):56‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host‐bacterial mutualism in the human intestine. Science. 2005;307(5717):1915‐1920. [DOI] [PubMed] [Google Scholar]
- 3. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity‐associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027‐1031. [DOI] [PubMed] [Google Scholar]
- 4. Koliada A, Syzenko G, Moseiko V, et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017;17(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Depommier C, Everard A, Druart C, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof‐of‐concept exploratory study. Nat Med. 2019;25(7):1096‐1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dao MC, Everard A, Aron‐Wisnewsky J, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65(3):426‐436. [DOI] [PubMed] [Google Scholar]
- 7. Solito A, Bozzi Cionci N, Calgaro M, et al. Supplementation with Bifidobacterium breve BR03 and B632 strains improved insulin sensitivity in children and adolescents with obesity in a cross‐over, randomized double‐blind placebo‐controlled trial. Clin Nutr. 2021;40(7):4585‐4594. [DOI] [PubMed] [Google Scholar]
- 8. Liu R, Hong J, Xu X, et al. Gut microbiome and serum metabolome alterations in obesity and after weight‐loss intervention. Nat Med. 2017;23(7):859‐868. [DOI] [PubMed] [Google Scholar]
- 9. Qin J, Li Y, Cai Z, et al. A metagenome‐wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55‐60. [DOI] [PubMed] [Google Scholar]
- 10. Zhao L, Zhang F, Ding X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359(6380):1151‐1156. [DOI] [PubMed] [Google Scholar]
- 11. Counillon L, Bouret Y, Marchiq I, Pouysségur J. Na(+)/H(+) antiporter (NHE1) and lactate/H(+) symporters (MCTs) in pH homeostasis and cancer metabolism. Biochim Biophys Acta. 2016;1863(10):2465‐2480. [DOI] [PubMed] [Google Scholar]
- 12. Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short‐chain fatty acids in health and disease. Adv Immunol. 2014;121:91‐119. [DOI] [PubMed] [Google Scholar]
- 13. Villodre Tudela C, Boudry C, Stumpff F, et al. Down‐regulation of monocarboxylate transporter 1 (MCT1) gene expression in the colon of piglets is linked to bacterial protein fermentation and pro‐inflammatory cytokine‐mediated signalling. Br J Nutr. 2015;113(4):610‐617. [DOI] [PubMed] [Google Scholar]
- 14. Ferrer‐Picón E, Dotti I, Corraliza AM, et al. Intestinal inflammation modulates the epithelial response to butyrate in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2020;26(1):43‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Louis P, Scott KP, Duncan SH, Flint HJ. Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbiol. 2007;102(5):1197‐1208. [DOI] [PubMed] [Google Scholar]
- 16. Miller TL, Wolin MJ. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl Environ Microbiol. 1996;62(5):1589‐1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Flint HJ, Duncan SH, Scott KP, Louis P. Links between diet, gut microbiota composition and gut metabolism. Proc Nutr Soc. 2015;74(1):13‐22. [DOI] [PubMed] [Google Scholar]
- 18. Ríos‐Covián D, Ruas‐Madiedo P, Margolles A, Gueimonde M, de Los Reyes‐Gavilán CG, Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. 2016;7:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Belenguer A, Duncan SH, Calder AG, et al. Two routes of metabolic cross‐feeding between Bifidobacterium adolescentis and butyrate‐producing anaerobes from the human gut. Appl Environ Microbiol. 2006;72(5):3593‐3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate‐producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 21. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li G, Xie C, Lu S, et al. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. 2017;26(4):672‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment‐naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23(7):850‐858. [DOI] [PubMed] [Google Scholar]
- 24. Mueller NT, Differding MK, Zhang M, et al. Metformin affects gut microbiome composition and function and circulating short‐chain fatty acids: a randomized trial. Diabetes Care. 2021;44(7):1462‐1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Palacios T, Vitetta L, Coulson S, et al. Targeting the intestinal microbiota to prevent type 2 diabetes and enhance the effect of metformin on glycaemia: a randomised controlled pilot study. Nutrients. 2020;12(7):2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Layden BT, Yalamanchi SK, Wolever TM, Dunaif A, Lowe WL. Negative association of acetate with visceral adipose tissue and insulin levels. Diabetes Metab Syndr Obes. 2012;5:49‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sanna S, van Zuydam NR, Mahajan A, et al. Causal relationships among the gut microbiome, short‐chain fatty acids and metabolic diseases. Nat Genet. 2019;51(4):600‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vitale M, Giacco R, Laiola M, et al. Acute and chronic improvement in postprandial glucose metabolism by a diet resembling the traditional Mediterranean dietary pattern: can SCFAs play a role? Clin Nutr. 2021;40(2):428‐437. [DOI] [PubMed] [Google Scholar]
- 29. Makki K, Deehan EC, Walter J, Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23(6):705‐715. [DOI] [PubMed] [Google Scholar]
- 30. Chambers ES, Viardot A, Psichas A, et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64(11):1744‐1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zou J, Chassaing B, Singh V, et al. Fiber‐mediated nourishment of gut microbiota protects against diet‐induced obesity by restoring IL‐22‐mediated colonic health. Cell Host Microbe. 2018;23(1):41‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hong J, Jia Y, Pan S, et al. Butyrate alleviates high fat diet‐induced obesity through activation of adiponectin‐mediated pathway and stimulation of mitochondrial function in the skeletal muscle of mice. Oncotarget. 2016;7(35):56071‐56082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yadav H, Lee J‐H, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate‐induced GLP‐1 hormone secretion. J Biol Chem. 2013;288(35):25088‐25097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. den Besten G, Bleeker A, Gerding A, et al. Short‐chain fatty acids protect against high‐fat diet‐induced obesity via a PPARγ‐dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64(7):2398‐2408. [DOI] [PubMed] [Google Scholar]
- 35. Huang Y, Gao S, Chen J, Albrecht E, Zhao R, Yang X. Maternal butyrate supplementation induces insulin resistance associated with enhanced intramuscular fat deposition in the offspring. Oncotarget. 2017;8(8):13073‐13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jia W, Wei M, Rajani C, Zheng X. Targeting the alternative bile acid synthetic pathway for metabolic diseases. Protein Cell. 2021;12(5):411‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wahlström A, Sayin SI, Marschall H‐U, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24(1):41‐50. [DOI] [PubMed] [Google Scholar]
- 38. Winston JA, Theriot CM. Diversification of host bile acids by members of the gut microbiota. Gut Microbes. 2020;11(2):158‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ridlon JM, Kang D‐J, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47(2):241‐259. [DOI] [PubMed] [Google Scholar]
- 40. Islam KBMS, Fukiya S, Hagio M, et al. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 2011;141(5):1773‐1781. [DOI] [PubMed] [Google Scholar]
- 41. Long SL, Gahan CGM, Joyce SA. Interactions between gut bacteria and bile in health and disease. Mol Aspects Med. 2017;56:54‐65. [DOI] [PubMed] [Google Scholar]
- 42. Grüner N, Mattner J. Bile acids and microbiota: multifaceted and versatile regulators of the liver–gut axis. Int J Mol Sci. 2021;22(3):1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li R, Andreu‐Sánchez S, Kuipers F, Fu J. Gut microbiome and bile acids in obesity‐related diseases. Best Pract Res Clin Endocrinol Metab. 2021;35(3):101493. [DOI] [PubMed] [Google Scholar]
- 44. Vincent RP, Omar S, Ghozlan S, et al. Higher circulating bile acid concentrations in obese patients with type 2 diabetes. Ann Clin Biochem. 2013;50(pt 4):360‐364. [DOI] [PubMed] [Google Scholar]
- 45. Wang S, Deng Y, Xie X, et al. Plasma bile acid changes in type 2 diabetes correlated with insulin secretion in two‐step hyperglycemic clamp. J Diabetes. 2018;10(11):874‐885. [DOI] [PubMed] [Google Scholar]
- 46. Chávez‐Talavera O, Haas J, Grzych G, Tailleux A, Staels B. Bile acid alterations in nonalcoholic fatty liver disease, obesity, insulin resistance and type 2 diabetes: what do the human studies tell? Curr Opin Lipidol. 2019;30(3):244‐254. [DOI] [PubMed] [Google Scholar]
- 47. Lin H, An Y, Tang H, Wang Y. Alterations of bile acids and gut microbiota in obesity induced by high fat diet in rat model. J Agric Food Chem. 2019;67(13):3624‐3632. [DOI] [PubMed] [Google Scholar]
- 48. Wei M, Huang F, Zhao L, et al. A dysregulated bile acid‐gut microbiota axis contributes to obesity susceptibility. EBioMedicine. 2020;55:102766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang K, Liao M, Zhou N, et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 2019;26(1):222‐235. [DOI] [PubMed] [Google Scholar]
- 50. de Siqueira Cardinelli C, Torrinhas RS, Sala P, et al. Fecal bile acid profile after Roux‐en‐Y gastric bypass and its association with the remission of type 2 diabetes in obese women: a preliminary study. Clin Nutr. 2019;38(6):2906‐2912. [DOI] [PubMed] [Google Scholar]
- 51. Ocaña‐Wilhelmi L, Martín‐Núñez GM, Ruiz‐Limón P, et al. Gut microbiota metabolism of bile acids could contribute to the bariatric surgery improvements in extreme obesity. Metabolites. 2021;11(11):733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Martineau MG, Raker C, Dixon PH, et al. The metabolic profile of intrahepatic cholestasis of pregnancy is associated with impaired glucose tolerance, dyslipidemia, and increased fetal growth. Diabetes Care. 2015;38(2):243‐248. [DOI] [PubMed] [Google Scholar]
- 53. Park YH, Kim JG, Shin YW, Kim SH, Whang KY. Effect of dietary inclusion of Lactobacillus acidophilus ATCC 43121 on cholesterol metabolism in rats. J Microbiol Biotechnol. 2007;17(4):655‐662. [PubMed] [Google Scholar]
- 54. Juárez‐Fernández M, Porras D, Petrov P, et al. The synbiotic combination of and quercetin ameliorates early obesity and NAFLD through gut microbiota reshaping and bile acid metabolism modulation. Antioxidants. 2021;10(12):2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hibberd AA, Yde CC, Ziegler ML, et al. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef Microbes. 2019;10(2):121‐135. [DOI] [PubMed] [Google Scholar]
- 56. Quan L‐H, Zhang C, Dong M, et al. Myristoleic acid produced by enterococci reduces obesity through brown adipose tissue activation. Gut. 2020;69(7):1239‐1247. [DOI] [PubMed] [Google Scholar]
- 57. Pujo J, Petitfils C, Le Faouder P, et al. Bacteria‐derived long chain fatty acid exhibits anti‐inflammatory properties in colitis. Gut. 2021;70(6):1088‐1097. [DOI] [PubMed] [Google Scholar]
- 58. Machate DJ, Figueiredo PS, Marcelino G, et al. Fatty acid diets: regulation of gut microbiota composition and obesity and its related metabolic dysbiosis. Int J Mol Sci. 2020;21(11):4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Krautkramer KA, Fan J, Bäckhed F. Gut microbial metabolites as multi‐kingdom intermediates. Nat Rev Microbiol. 2021;19(2):77‐94. [DOI] [PubMed] [Google Scholar]
- 60. Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23(6):716‐724. [DOI] [PubMed] [Google Scholar]
- 61. Cussotto S, Delgado I, Anesi A, et al. Tryptophan metabolic pathways are altered in obesity and are associated with systemic inflammation. Front Immunol. 2020;11:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Menni C, Hernandez MM, Vital M, Mohney RP, Spector TD, Valdes AM. Circulating levels of the anti‐oxidant indoleproprionic acid are associated with higher gut microbiome diversity. Gut Microbes. 2019;10(6):688‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu Y, Yang K, Jia Y, et al. Gut microbiome alterations in high‐fat‐diet‐fed mice are associated with antibiotic tolerance. Nat Microbiol. 2021;6(7):874‐884. [DOI] [PubMed] [Google Scholar]
- 64. Behera J, Ison J, Voor MJ, Tyagi N. Probiotics stimulate bone formation in obese mice via histone methylations. Theranostics. 2021;11(17):8605‐8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tuomainen M, Lindström J, Lehtonen M, et al. Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low‐grade inflammation in high‐risk individuals. Nutr Diabetes. 2018;8(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hubbard TD, Murray IA, Perdew GH. Indole and tryptophan metabolism: endogenous and dietary routes to Ah receptor activation. Drug Metab Dispos. 2015;43(10):1522‐1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gao J, Xu K, Liu H, et al. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol. 2018;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Su X, Zhang M, Qi H, et al. Gut microbiota‐derived metabolite 3‐idoleacetic acid together with LPS induces IL‐35 B cell generation. Microbiome. 2022;10(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Depommier C, Everard A, Druart C, et al. Serum metabolite profiling yields insights into health promoting effect of A. muciniphila in human volunteers with a metabolic syndrome. Gut Microbes. 2021;13(1):1994270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wu T, Sun M, Liu R, et al. Remodeled and phosphatidylserine levels and ameliorated intestinal disorders and liver metabolic abnormalities induced by high‐fat diet. J Agric Food Chem. 2020;68(16):4632‐4640. [DOI] [PubMed] [Google Scholar]
- 71. Jennis M, Cavanaugh CR, Leo GC, Mabus JR, Lenhard J, Hornby PJ. Microbiota‐derived tryptophan indoles increase after gastric bypass surgery and reduce intestinal permeability in vitro and in vivo. Neurogastroenterol Motil. 2018;30(2). [DOI] [PubMed] [Google Scholar]
- 72. Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci U S A. 2012;109(52):21307‐21312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shan Z, Sun T, Huang H, et al. Association between microbiota‐dependent metabolite trimethylamine‐oxide and type 2 diabetes. Am J Clin Nutr. 2017;106(3):888‐894. [DOI] [PubMed] [Google Scholar]
- 74. Dehghan P, Farhangi MA, Nikniaz L, Nikniaz Z, Asghari‐Jafarabadi M. Gut microbiota‐derived metabolite trimethylamine N‐oxide (TMAO) potentially increases the risk of obesity in adults: an exploratory systematic review and dose–response meta‐analysis. Obes Rev. 2020;21(5):e12993. [DOI] [PubMed] [Google Scholar]
- 75. Won E‐Y, Yoon M‐K, Kim S‐W, et al. Gender‐specific metabolomic profiling of obesity in leptin‐deficient ob/ob mice by 1H NMR spectroscopy. PLoS One. 2013;8(10):e75998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Miao J, Ling AV, Manthena PV, et al. Flavin‐containing monooxygenase 3 as a potential player in diabetes‐associated atherosclerosis. Nat Commun. 2015;6:6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Svingen GFT, Schartum‐Hansen H, Pedersen ER, et al. Prospective associations of systemic and urinary choline metabolites with incident type 2 diabetes. Clin Chem. 2016;62(5):755‐765. [DOI] [PubMed] [Google Scholar]
- 78. Yoo W, Zieba JK, Foegeding NJ, et al. High‐fat diet‐induced colonocyte dysfunction escalates microbiota‐derived trimethylamine‐oxide. Science. 2021;373(6556):813‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shi Q, Wang Q, Zhong H, et al. Roux‐en‐Y gastric bypass improved insulin resistance via alteration of the human gut microbiome and alleviation of endotoxemia. Biomed Res Int. 2021;2021:5554991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Heianza Y, Sun D, Smith SR, Bray GA, Sacks FM, Qi L. Changes in gut microbiota‐related metabolites and long‐term successful weight loss in response to weight‐loss diets: the POUNDS Lost Trial. Diabetes Care. 2018;41(3):413‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Argyridou S, Davies MJ, Biddle GJH, et al. Evaluation of an 8‐week vegan diet on plasma trimethylamine‐N‐oxide and postchallenge glucose in adults with dysglycemia or obesity. J Nutr. 2021;151(7):1844‐1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Martínez‐del Campo A, Bodea S, Hamer HA, et al. Characterization and detection of a widely distributed gene cluster that predicts anaerobic choline utilization by human gut bacteria. mBio. 2015;6(2):e00042‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhu Y, Jameson E, Crosatti M, et al. Carnitine metabolism to trimethylamine by an unusual Rieske‐type oxygenase from human microbiota. Proc Natl Acad Sci U S A. 2014;111(11):4268‐4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wu W‐K, Chen C‐C, Liu P‐Y, et al. Identification of TMAO‐producer phenotype and host‐diet‐gut dysbiosis by carnitine challenge test in human and germ‐free mice. Gut. 2019;68(8):1439‐1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Schugar RC, Gliniak CM, Osborn LJ, et al. Gut microbe‐targeted choline trimethylamine lyase inhibition improves obesity via rewiring of host circadian rhythms. Elife. 2022;11:e63998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Steinke I, Ghanei N, Govindarajulu M, Yoo S, Zhong J, Amin RH. Drug discovery and development of novel therapeutics for inhibiting TMAO in models of atherosclerosis and diabetes. Front Physiol. 2020;11:567899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jones ML, Tomaro‐Duchesneau C, Prakash S. The gut microbiome, probiotics, bile acids axis, and human health. Trends Microbiol. 2014;22(6):306‐308. [DOI] [PubMed] [Google Scholar]
- 88. Liu KH, Owens JA, Saeedi B, et al. Microbial metabolite delta‐valerobetaine is a diet‐dependent obesogen. Nat Metab. 2021;3(12):1694‐1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ringseis R, Keller J, Eder K. Role of carnitine in the regulation of glucose homeostasis and insulin sensitivity: evidence from in vivo and in vitro studies with carnitine supplementation and carnitine deficiency. Eur J Nutr. 2012;51(1):1‐18. [DOI] [PubMed] [Google Scholar]
- 90. Molinaro A, Bel Lassen P, Henricsson M, et al. Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nat Commun. 2020;11(1):5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wilmanski T, Rappaport N, Earls JC, et al. Blood metabolome predicts gut microbiome α‐diversity in humans. Nat Biotechnol. 2019;37(10):1217‐1228. [DOI] [PubMed] [Google Scholar]
- 92. Scheithauer TPM, Rampanelli E, Nieuwdorp M, et al. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front Immunol. 2020;11:571731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kumar DP, Rajagopal S, Mahavadi S, et al. Activation of transmembrane bile acid receptor TGR5 stimulates insulin secretion in pancreatic β cells. Biochem Biophys Res Commun. 2012;427(3):600‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Brønden A, Albér A, Rohde U, et al. The bile acid‐sequestering resin sevelamer eliminates the acute GLP‐1 stimulatory effect of endogenously released bile acids in patients with type 2 diabetes. Diabetes Obes Metab. 2018;20(2):362‐369. [DOI] [PubMed] [Google Scholar]
- 95. Trabelsi M‐S, Daoudi M, Prawitt J, et al. Farnesoid X receptor inhibits glucagon‐like peptide‐1 production by enteroendocrine L cells. Nat Commun. 2015;6:7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tolhurst G, Heffron H, Lam YS, et al. Short‐chain fatty acids stimulate glucagon‐like peptide‐1 secretion via the G‐protein‐coupled receptor FFAR2. Diabetes. 2012;61(2):364‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Larraufie P, Martin‐Gallausiaux C, Lapaque N, et al. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci Rep. 2018;8(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Larraufie P, Doré J, Lapaque N, Blottière HM. TLR ligands and butyrate increase PYY expression through two distinct but inter‐regulated pathways. Cell Microbiol. 2017;19(2). [DOI] [PubMed] [Google Scholar]
- 99. Natividad JM, Agus A, Planchais J, et al. Impaired aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metab. 2018;28(5):737‐749.e4. [DOI] [PubMed] [Google Scholar]
- 100. Chimerel C, Emery E, Summers DK, Keyser U, Gribble FM, Reimann F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 2014;9(4):1202‐1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Castellanos‐Jankiewicz A, Guzmán‐Quevedo O, Fénelon VS, et al. Hypothalamic bile acid‐TGR5 signaling protects from obesity. Cell Metab. 2021;33(7):1483‐1492.e10. [DOI] [PubMed] [Google Scholar]
- 102. Frost G, Sleeth ML, Sahuri‐Arisoylu M, et al. The short‐chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Li Z, Yi C‐X, Katiraei S, et al. Butyrate reduces appetite and activates brown adipose tissue via the gut–brain neural circuit. Gut. 2018;67(7):1269‐1279. [DOI] [PubMed] [Google Scholar]
- 104. Liu J‐L, Segovia I, Yuan X‐L, Gao Z‐H. Controversial roles of gut microbiota‐derived short‐chain fatty acids (SCFAs) on pancreatic β‐cell growth and insulin secretion. Int J Mol Sci. 2020;21(3):910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. McNelis JC, Lee YS, Mayoral R, et al. GPR43 potentiates β‐cell function in obesity. Diabetes. 2015;64(9):3203‐3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kimura I, Ozawa K, Inoue D, et al. The gut microbiota suppresses insulin‐mediated fat accumulation via the short‐chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Priyadarshini M, Wicksteed B, Schiltz GE, Gilchrist A, Layden BT. SCFA receptors in pancreatic β cells: novel diabetes targets? Trends Endocrinol Metab. 2016;27(9):653‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Vettorazzi JF, Ribeiro RA, Borck PC, et al. The bile acid TUDCA increases glucose‐induced insulin secretion via the cAMP/PKA pathway in pancreatic beta cells. Metabolism. 2016;65(3):54‐63. [DOI] [PubMed] [Google Scholar]
- 109. Maczewsky J, Kaiser J, Gresch A, et al. TGR5 activation promotes stimulus‐secretion coupling of pancreatic β‐cells via a PKA‐dependent pathway. Diabetes. 2019;68(2):324‐336. [DOI] [PubMed] [Google Scholar]
- 110. Düfer M, Hörth K, Wagner R, et al. Bile acids acutely stimulate insulin secretion of mouse β‐cells via farnesoid X receptor activation and K(ATP) channel inhibition. Diabetes. 2012;61(6):1479‐1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Baggio LL, Drucker DJ. Biology of incretins: GLP‐1 and GIP. Gastroenterology. 2007;132(6):2131‐2157. [DOI] [PubMed] [Google Scholar]
- 112. Müller TD, Finan B, Bloom SR, et al. Glucagon‐like peptide 1 (GLP‐1). Mol Metab. 2019;30:72‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Montanari T, Pošćić N, Colitti M. Factors involved in white‐to‐brown adipose tissue conversion and in thermogenesis: a review. Obes Rev. 2017;18(5):495‐513. [DOI] [PubMed] [Google Scholar]
- 114. Zietak M, Kozak LP. Bile acids induce uncoupling protein 1‐dependent thermogenesis and stimulate energy expenditure at thermoneutrality in mice. Am J Physiol Endocrinol Metab. 2016;310(5):E346‐E354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Broeders EPM, Nascimento EBM, Havekes B, et al. The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab. 2015;22(3):418‐426. [DOI] [PubMed] [Google Scholar]
- 116. Herz CT, Kulterer OC, Prager M, et al. Characterization of endogenous bile acid composition in individuals with cold‐activated brown adipose tissue. Mol Cell Endocrinol. 2021;536:111403. [DOI] [PubMed] [Google Scholar]
- 117. Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484‐489. [DOI] [PubMed] [Google Scholar]
- 118. Chen Y‐S, Liu H‐M, Lee T‐Y. Ursodeoxycholic acid regulates hepatic energy homeostasis and white adipose tissue macrophages polarization in leptin‐deficiency obese mice. Cells. 2019;8(3):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kim J, Okla M, Erickson A, Carr T, Natarajan SK, Chung S. Eicosapentaenoic acid potentiates brown thermogenesis through FFAR4‐dependent up‐regulation of miR‐30b and miR‐378. J Biol Chem. 2016;291(39):20551‐20562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty‐acid‐dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151(2):400‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Schugar RC, Shih DM, Warrier M, et al. The TMAO‐producing enzyme flavin‐containing monooxygenase 3 regulates obesity and the beiging of white adipose tissue. Cell Rep. 2017;20(1):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Schugar RC, Willard B, Wang Z, Brown JM. Postprandial gut microbiota‐driven choline metabolism links dietary cues to adipose tissue dysfunction. Adipocyte. 2018;7(1):49‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rumberger JM, Arch JRS, Green A. Butyrate and other short‐chain fatty acids increase the rate of lipolysis in 3T3‐L1 adipocytes. PeerJ. 2014;2:e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sa A‐L, Roelofsen H, Rezaee F, et al. Propionic acid affects immune status and metabolism in adipose tissue from overweight subjects. Eur J Clin Invest. 2012;42(4):357‐364. [DOI] [PubMed] [Google Scholar]
- 125. Li G, Yao W, Jiang H. Short‐chain fatty acids enhance adipocyte differentiation in the stromal vascular fraction of porcine adipose tissue. J Nutr. 2014;144(12):1887‐1895. [DOI] [PubMed] [Google Scholar]
- 126. Zhang Y, Fang F, Goldstein JL, Brown MS, Zhao T‐J. Reduced autophagy in livers of fasted, fat‐depleted, ghrelin‐deficient mice: reversal by growth hormone. Proc Natl Acad Sci U S A. 2015;112(4):1226‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kato S‐I, Sato K, Chida H, et al. Effects of Na‐butyrate supplementation in milk formula on plasma concentrations of GH and insulin, and on rumen papilla development in calves. J Endocrinol. 2011;211(3):241‐248. [DOI] [PubMed] [Google Scholar]
- 128. Miletta MC, Petkovic V, Eblé A, Ammann RA, Flück CE, Mullis P‐E. Butyrate increases intracellular calcium levels and enhances growth hormone release from rat anterior pituitary cells via the G‐protein‐coupled receptors GPR41 and 43. PLoS One. 2014;9(10):e107388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. 2021;320(3):C375‐C391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Al‐Attas OS, Al‐Daghri NM, Al‐Rubeaan K, et al. Changes in endotoxin levels in T2DM subjects on anti‐diabetic therapies. Cardiovasc Diabetol. 2009;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Elamin EE, Masclee AA, Dekker J, Pieters H‐J, Jonkers DM. Short‐chain fatty acids activate AMP‐activated protein kinase and ameliorate ethanol‐induced intestinal barrier dysfunction in CaCo‐2 cell monolayers. J Nutr. 2013;143(12):1872‐1881. [DOI] [PubMed] [Google Scholar]
- 132. Venkatesh M, Mukherjee S, Wang H, et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll‐like receptor 4. Immunity. 2014;41(2):296‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Maurizi G, Della Guardia L, Maurizi A, Poloni A. Adipocytes properties and crosstalk with immune system in obesity‐related inflammation. J Cell Physiol. 2018;233(1):88‐97. [DOI] [PubMed] [Google Scholar]
- 134. Tedelind S, Westberg F, Kjerrulf M, Vidal A. Anti‐inflammatory properties of the short‐chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol. 2007;13(20):2826‐2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Shen S, Prame Kumar K, Wen SW, et al. Deficiency of dietary fiber modulates gut microbiota composition, neutrophil recruitment and worsens experimental colitis. Front Immunol. 2021;12:619366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Biagioli M, Carino A, Cipriani S, et al. The bile acid receptor GPBAR1 regulates the M1/M2 phenotype of intestinal macrophages and activation of GPBAR1 rescues mice from murine colitis. J Immunol. 2017;199(2):718‐733. [DOI] [PubMed] [Google Scholar]
- 137. Pols TWH, Nomura M, Harach T, et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 2011;14(6):747‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Guo C, Xie S, Chi Z, et al. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity. 2016;45(4):802‐816. [DOI] [PubMed] [Google Scholar]
- 139. Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914‐920. [DOI] [PubMed] [Google Scholar]
- 140. Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short‐chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T‐cell generation. Nature. 2013;504(7480):451‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Song X, Sun X, Oh SF, et al. Microbial bile acid metabolites modulate gut RORγ regulatory T cell homeostasis. Nature. 2020;577(7790):410‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Hang S, Paik D, Yao L, et al. Bile acid metabolites control T17 and T cell differentiation. Nature. 2019;576(7785):143‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Virtue AT, McCright SJ, Wright JM, et al. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci Transl Med. 2019;11(496):eaav1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Zelante T, Iannitti RG, Cunha C, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin‐22. Immunity. 2013;39(2):372‐385. [DOI] [PubMed] [Google Scholar]
- 146. Yang W, Yu T, Huang X, et al. Intestinal microbiota‐derived short‐chain fatty acids regulation of immune cell IL‐22 production and gut immunity. Nat Commun. 2020;11(1):4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Sánchez‐Alcoholado L, Ordóñez R, Otero A, et al. Gut microbiota‐mediated inflammation and gut permeability in patients with obesity and colorectal cancer. Int J Mol Sci. 2020;21(18):6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Robert M, Espalieu P, Pelascini E, et al. Efficacy and safety of one anastomosis gastric bypass versus Roux‐en‐Y gastric bypass for obesity (YOMEGA): a multicentre, randomised, open‐label, non‐inferiority trial. Lancet. 2019;393(10178):1299‐1309. [DOI] [PubMed] [Google Scholar]
- 149. Courcoulas AP, Gallagher JW, Neiberg RH, et al. Bariatric surgery vs lifestyle intervention for diabetes treatment: 5‐year outcomes from a randomized trial. J Clin Endocrinol Metab. 2020;105(3):866‐876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Lau DCW, Erichsen L, Francisco AM, et al. Once‐weekly cagrilintide for weight management in people with overweight and obesity: a multicentre, randomised, double‐blind, placebo‐controlled and active‐controlled, dose‐finding phase 2 trial. Lancet. 2021;398(10317):2160‐2172. [DOI] [PubMed] [Google Scholar]
- 151. Wilson BC, Vatanen T, Jayasinghe TN, et al. Strain engraftment competition and functional augmentation in a multi‐donor fecal microbiota transplantation trial for obesity. Microbiome. 2021;9(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Canfora EE, van der Beek CM, Jocken JWE, et al. Colonic infusions of short‐chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Sci Rep. 2017;7(1):2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Calderon G, McRae A, Rievaj J, et al. Ileo‐colonic delivery of conjugated bile acids improves glucose homeostasis via colonic GLP‐1‐producing enteroendocrine cells in human obesity and diabetes. EBioMedicine. 2020;55:102759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Leong KSW, Jayasinghe TN, Wilson BC, et al. Effects of fecal microbiome transfer in adolescents with obesity: the gut bugs randomized controlled trial. JAMA Netw Open. 2020;3(12):e2030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Yu EW, Gao L, Stastka P, et al. Fecal microbiota transplantation for the improvement of metabolism in obesity: the FMT‐TRIM double‐blind placebo‐controlled pilot trial. PLoS Med. 2020;17(3):e1003051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Allegretti JR, Kassam Z, Mullish BH, et al. Effects of fecal microbiota transplantation with oral capsules in obese patients. Clin Gastroenterol Hepatol. 2020;18(4):855‐863.e2. [DOI] [PubMed] [Google Scholar]
- 157. Kootte RS, Levin E, Salojärvi J, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017;26(4):611‐619.e6. [DOI] [PubMed] [Google Scholar]
- 158. Mocanu V, Zhang Z, Deehan EC, et al. Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: a randomized double‐blind, placebo‐controlled phase 2 trial. Nat Med. 2021;27(7):1272‐1279. [DOI] [PubMed] [Google Scholar]
- 159. Ng SC, Xu Z, Mak JWY, et al. Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: a 24‐week, double‐blind, randomised controlled trial. Gut. 2022;71(4):716‐723. [DOI] [PubMed] [Google Scholar]
- 160. Rinott E, Youngster I, Yaskolka Meir A, et al. Effects of diet‐modulated autologous fecal microbiota transplantation on weight regain. Gastroenterology. 2021;160(1):158‐173.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Şanlier N, Gökcen BB, Sezgin AC. Health benefits of fermented foods. Crit Rev Food Sci Nutr. 2019;59(3):506‐527. [DOI] [PubMed] [Google Scholar]
- 162. Pei R, Martin DA, DiMarco DM, Bolling BW. Evidence for the effects of yogurt on gut health and obesity. Crit Rev Food Sci Nutr. 2017;57(8):1569‐1583. [DOI] [PubMed] [Google Scholar]
- 163. Hidalgo‐Cantabrana C, Delgado S, Ruiz L, Ruas‐Madiedo P, Sánchez B, Margolles A. Bifidobacteria and their health‐promoting effects. Microbiol Spectr. 2017;5(3). [DOI] [PubMed] [Google Scholar]
- 164. Seddik HA, Bendali F, Gancel F, Fliss I, Spano G, Drider D. Lactobacillus plantarum and its probiotic and food potentialities. Probiotics Antimicrob Proteins. 2017;9(2):111‐122. [DOI] [PubMed] [Google Scholar]
- 165. Tsai Y‐L, Lin T‐L, Chang C‐J, et al. Probiotics, prebiotics and amelioration of diseases. J Biomed Sci. 2019;26(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Vallianou N, Stratigou T, Christodoulatos GS, Tsigalou C, Dalamaga M. Probiotics, prebiotics, synbiotics, postbiotics, and obesity: current evidence, controversies, and perspectives. Curr Obes Rep. 2020;9(3):179‐192. [DOI] [PubMed] [Google Scholar]
- 167. Abenavoli L, Scarpellini E, Colica C, et al. Gut microbiota and obesity: a role for probiotics. Nutrients. 2019;11(11):2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Everard A, Belzer C, Geurts L, et al. Cross‐talk between Akkermansia muciniphila and intestinal epithelium controls diet‐induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066‐9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Plovier H, Everard A, Druart C, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23(1):107‐113. [DOI] [PubMed] [Google Scholar]
- 170. Depommier C, Vitale RM, Iannotti FA, et al. Beneficial effects of are not associated with major changes in the circulating endocannabinoidome but linked to higher mono‐palmitoyl‐glycerol levels as new PPARα agonists. Cells. 2021;10(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Guman MSS, Haal S, Maurits de Brauw L, et al. Factors associated with adherence to ursodeoxycholic acid or placebo in patients after bariatric surgery. Surg Obes Relat Dis. 2022;18(6):755‐761. [DOI] [PubMed] [Google Scholar]
- 172. Haal S, Guman MSS, Boerlage TCC, et al. Ursodeoxycholic acid for the prevention of symptomatic gallstone disease after bariatric surgery (UPGRADE): a multicentre, double‐blind, randomised, placebo‐controlled superiority trial. Lancet Gastroenterol Hepatol. 2021;6(12). [DOI] [PubMed] [Google Scholar]
- 173. Pizza F, D'Antonio D, Lucido FS, et al. The role of ursodeoxycholic acid (UDCA) in cholelithiasis management after one anastomosis gastric bypass (OAGB) for morbid obesity: results of a monocentric randomized controlled trial. Obes Surg. 2020;30(11):4315‐4324. [DOI] [PubMed] [Google Scholar]
- 174. Arnoriaga‐Rodríguez M, Mayneris‐Perxachs J, Contreras‐Rodríguez O, et al. Obesity‐associated deficits in inhibitory control are phenocopied to mice through gut microbiota changes in one‐carbon and aromatic amino acids metabolic pathways. Gut. 2021;70(12):2283‐2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Rasmussen TS, Mentzel CMJ, Kot W, et al. Faecal virome transplantation decreases symptoms of type 2 diabetes and obesity in a murine model. Gut. 2020;69(12):2122‐2130. [DOI] [PubMed] [Google Scholar]
- 176. Trikha SRJ, Lee DM, Ecton KE, et al. Transplantation of an obesity‐associated human gut microbiota to mice induces vascular dysfunction and glucose intolerance. Gut Microbes. 2021;13(1):1940791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Zoll J, Read MN, Heywood SE, et al. Fecal microbiota transplantation from high caloric‐fed donors alters glucose metabolism in recipient mice, independently of adiposity or exercise status. Am J Physiol Endocrinol Metab. 2020;319(1):E203‐E216. [DOI] [PubMed] [Google Scholar]
- 178. Tonucci LB, Olbrich Dos Santos KM, Licursi de Oliveira L, Rocha Ribeiro SM, Duarte Martino HS. Clinical application of probiotics in type 2 diabetes mellitus: a randomized, double‐blind, placebo‐controlled study. Clin Nutr. 2017;36(1):85‐92. [DOI] [PubMed] [Google Scholar]
- 179. Shin NR, Gu N, Choi HS, Kim H. Combined effects of with metformin on glucose tolerance of patients with type 2 diabetes via gut microbiota modulation. Am J Physiol Endocrinol Metab. 2020;318(1):E52‐E61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


