Abstract
Background
Postsurgical hypoparathyroidism (PH) is the most frequent complication after thyroid surgery. The aim of this systematic review and meta-analysis is to summarize a unifying definition of PH and to elucidate the best possible approach for early detection of PH.
Methods
A systematic review of the literature according to the PICO framework using Embase, PUBMED and the Cochrane library was carried out on 1 December 2021 followed by analysis for risk of bias, data extraction and meta-analysis. All studies addressing the definition of postoperative hypoparathyroidism and/or diagnostic approaches for early detection and diagnosis were included. Case reports, commentaries, non-English articles, book chapters and pilot studies and reviews were excluded.
Results
From 13 704 articles, 188 articles were eligible for inclusion and further analysis. These articles provided heterogeneous definitions of PH. Meta-analysis revealed that postoperative measurements of parathormone (PTH) levels have a higher sensitivity and specificity than intraoperative PTH measurements to predict PH after thyroid surgery. None of the timeframes analysed after surgery within the first postoperative day (POD1) was superior to predict the onset of PH. PTH levels of less than 15 pg/ml and less than 10 pg/ml are both reliable threshold levels to predict the postoperative onset of PH. A relative reduction of mean(s.d.) PTH levels from pre- to postoperative values of 73 (standard deviation 11) per cent may also be predictive for the development of PH. The estimation of calcium levels on POD1 are recommended.
Conclusion
PH is best defined as an undetectable or inappropriately low postoperative PTH level in the context of hypocalcaemia with or without hypocalcaemic symptoms. PTH levels should be measured after surgery within 24 h. Both threshold levels below 10 and 15 pg/ml or relative loss of PTH before/after thyroid surgery are reliable to predict the onset of PH.
Actual and comprehensive overview of the definition of postsurgical hypoparathyroidism and meta-analysis on when and how to detect postsurgical hypoparathyroidism. Postsurgical measurements of PTH levels are more sensitive and more specific than intraoperative PTH measurements to predict postsurgical hypoparathyroidism. The end of the operation until 24 h after surgery are the best time points to measure PTH levels to predict postsurgical hypoparathyroidism. PTH levels below 15 and 10 pg/ml have a high sensitivity and specificity to predict the development of postsurgical hypoparathyroidism reliably.
Introduction
With a rate of 14–60 per cent, postsurgical hypoparathyroidism (PH) is the most frequent complication after thyroid surgery1–3. Although most patients seen with PH only have transient problems, there is still a significant number of patients (up to 33 per cent) suffering from persisting hypoparathyroidism (reduced parathyroid hormone (PTH) and calcium levels persisting more than 6 months after thyroid surgery)2,3. In a narrative review it has been proposed previously that the time dimension should be incorporated when describing PH. Based on this, PH includes the syndromes of postoperative parathyroid failure, protracted hypoparathyroidism and permanent hypoparathyroidism4.
The symptoms of hypoparathyroidism are extremely variable and range from no symptoms to mild numbness and tingling, muscle cramps, tetany, seizures and life-threatening laryngospasm and cardiac arrhythmia.
In most cases, the onset of PH is within the first 48 h after thyroid surgery5–8; however, it has also been reported that the first symptoms of hypocalcaemia begin much later, up to 64 h after surgery9–11. This has also been described as postoperative parathyroid failure4. In view of this, ongoing efforts to discharge patients 24–48 h after thyroid surgery can lead to significant danger for patients, as they will not receive adequate and timely therapeutic intervention if they develop symptoms later. This concern requires a standardized follow-up of patients after thyroid surgery and the earliest possible detection of PH. It would also be desirable to have reliable markers to predict the potential onset of PH to start a (preventive) therapeutic intervention before patients get symptomatic. This is important because a standard ‘blind’ substitution of calcium and vitamin D with the aim of preventing the onset of symptoms has been reported to be one of the main risk factors of developing PH, as the physiological trigger for PTH secretion is blocked12.
All these aspects have been addressed by numerous studies so far. Accordingly, there is a large body of literature focusing on how to diagnose PH at the earliest possible time point to decide whether therapeutic intervention is required; however, because even the definition of PH is heterogeneous and there are many different studies addressing this, there remains uncertainty about the appropriate approach for early recognition of PH.
A systematic analysis of the literature on postsurgical hypoparathyroidism was therefore conducted focusing on the following issues: the most common definitions of PH were systematically analysed and discussed. Next, the question of whether there is an ideal time point for early detection of PH or postoperative parathyroid failure respectively, was assessed. Finally, the best possible predictive approach for early recognition of PH was determined.
Materials and methods
Search strategy
A systematic review of the literature was conducted according to the PRISMA guidelines13. The review protocol was registered at Prospero (https://www.crd.york.ac.uk/); PROSPERO 2022 CRD42022303713.
To get a comprehensive overview of the existing body of literature, a systematic literature search of PubMed via MEDLINE, Embase and the Cochrane library electronic databases was performed on 1 December 2021. The timeframe of the literature search was from the overall start of documentation in the databases until 30 November 2021. A systemic analysis of the literature was conducted according to the PICO framework14. According to this, ‘patients after thyroid surgery’ were defined as the population and ‘postsurgical hypoparathyroidism’ as the phenomenon of interest, and ‘diagnostics’ as the context. All search terms were assigned into these three subgroups (Table S1). The words ‘AND’ and ‘OR’ were used as Boolean operators. To increase the sensitivity of the literature search, the following medical subject heading terms were included: ‘hypoparathyroidism’, ‘hypocalcemia’, ‘postoperative complications’, ‘postoperative period’, ‘thyroidectomy’, ‘parathyroid hormone’, ‘hemithyroidectomy’ and ‘subtotal thyroidectomy’. Again ‘AND’ and ‘OR’ were used as Boolean operators.
All studies with abstracts in the English language were included. Duplicates were removed by the literature organization program in addition to manual control. Two independent reviewers (K.N. and N.S.) performed the screening of titles and abstracts of all studies. Potentially relevant articles were reviewed in full to determine eligibility for inclusion. Data for meta-analysis were extracted by one author (K.N.) and double checked by the other authors (A.H. and N.S.). In case of missing/incomplete data, the study investigator was contacted for additional details. Any disagreement was discussed and solved by consensus among the authors.
Study selection criteria
All studies addressing the definition of PH and/or diagnostic approaches for early detection and diagnosis were included. Both, prospective and retrospective studies were included. Case reports, commentaries, non-English articles, book chapters and pilot studies and reviews were excluded. Conference proceedings and unpublished studies were included if they provided sufficient information. If two studies examined the same study population, the more recent study was included.
For meta-analyses, all studies were manually screened and compared regarding whether they displayed a comparable study design and equal outcome parameters. This is outlined in detail in the results for the respective topic addressed.
Data management, risk of bias assessment and statistical analysis
The literature organization was performed with Endnote20™ (Clarivate Analytics, Munich, Germany). Charts and tables were created with Microsoft® Word and Microsoft® PowerPoint (Microsoft, Redmond, Washington, USA), and RevMan5 (Cochrane Community). The studies included for meta-analysis were assessed for the risk of bias using the ROBINS-I tool15 (Table S2).
To compare PTH levels and calcium levels and to provide a comprehensive overview, units were adapted for PTH in pg/ml and for calcium in mmol/l. The calculation was performed using the calculator provided in unitslab.com.
Statistical analysis was performed with SPSS® version 26 (IBM, Armonk, New York, USA), RevMan5 and OpenMeta (Analyst)16. As a measure of effects, bivariate analysis for sensitivity and specificity with the corresponding 95 per cent confidence interval (c.i.) was calculated.
Results
The database search identified 13 704 articles. After removing duplications, 8850 articles were screened for eligibility and inclusion in the systematic review (Fig. 1). After exclusion of studies by title/abstract and full text screening, 188 articles were eligible for inclusion in this review. After this, all articles were analysed in depth according to the main foci of this review. This led to a variable number of studies included for the different subheadings, stated below. Analyses for risk of bias in the studies included in the meta-analyses are shown in Table S2.
Fig. 1.
PRISMA flowchart for the literature search
Definition of postsurgical hypoparathyroidism
According to the focus ‘definition of PH’, 188 articles identified in the database were assessed for eligibility. From these, 31 studies were excluded because hypoparathyroidism was not defined, or no clear definition was stated so that 157 articles including 29 346 patients were subject to further analysis.
As shown in Table 1, these studies could be assigned into four subgroups. The definitions of PH included reduced PTH levels only, hypocalcaemia only, reduced PTH or hypocalcaemia or a combination of both. In most cases, these groups could be subdivided into studies that included the presence or absence of symptoms to define the presence of PH. Taken together, this confirmed that there is a large heterogeneity of definitions of PH in the different studies. Therefore, direct systematic comparisons of the parameters discussed below are nearly impossible.
Table 1.
Studies identified with specific definitions of postsurgical hypoparathyroidism
| Definition criteria of postsurgical hypoparathyroidism | Number of patients included | References |
|---|---|---|
| Reduced PTH levels n = 3 | ||
| Biochemical alterations only | 461 | 17–19 |
| Hypocalcaemia AND reduced PTH levels n = 2 | ||
| Biochemical alterations only | 1172 | 20,21 |
| Hypocalcaemia OR reduced PTH levels n = 20 | ||
| Biochemical alterations only n = 8 | 2563 | 22–29 |
| Symptoms independent from biochemical hypocalcaemia n = 2 | 486 | 30,31 |
| Biochemical alterations and/or symptoms n = 10 | 2920 | 32–41 |
| Hypocalcaemia only n = 132 | ||
| Biochemical alterations only n = 42 | 6475 | 6,7,42–81 |
| Symptoms independent from hypocalcaemia n = 20 | 4258 | 82–101 |
| Hypocalcaemia and/or symptoms n = 63 | 9936 | 10,102–163 |
| Hypocalcaemia and symptoms n = 7 | 1075 | 164–170 |
The main criteria and the sub-definitions within these categories are listed in the first column; n indicates the number of studies found. Reduced PTH and calcium indicates levels below the level of normal; whether calcium levels referred to adjusted, ionized or total calcium was reported in the studies could not be identified in all studies so that they were taken together as ‘hypocalcaemia’. PTH, parathyroid hormone.
In addition, the lower limits of serum calcium levels seem slightly heterogeneous depending on the local laboratories171. Forty studies including 7718 patients defined hypocalcaemia according to the lower limit of normal of the local laboratories in which their measurements were carried out. This corresponds to the recommendation of the American Association of Clinical Endocrinology172. The majority of 52 studies including 11 504 patients, defined hypocalcaemia as a calcium level below the lower limit of 2.0 mmol/l (less than 8.0 mg/dl), which is in accordance with other recommendations8,173. It must be considered whether uncorrected serum calcium levels, albumin-corrected serum calcium levels or ionized calcium levels are the basis for the different studies to define hypocalcaemia. In addition, there is evidence that patients with calcium values less than 2.0 mmol/l may develop symptoms of hypocalcaemia102,174. In view of all these aspects, both approaches to define hypocalcaemia seem justified although they do not necessarily correspond to clinical symptoms.
On the other hand, the reduction of PTH levels is a good predictor for symptomatic hypoparathyroidism. It has been shown that it is more sensitive at detecting patients at risk and detecting them earlier because loss of PTH precedes biochemical hypocalcaemia82,103,104. Therefore, based on the literature search, the diagnosis should be predominately oriented on the early biochemical changes of PTH levels after surgery.
When addressing the clinical picture caused by hypoparathyroidism, a large inter-individual variety was found ranging from ‘no symptoms’ in patients with clear biochemical evidence for the presence of PH to a group of patients with ‘potentially life-threatening symptoms’ because of laryngospasm, muscle cramps or cardiac arrhythmia with biochemical changes that were mild at the time when symptoms started104,175. Therefore, it was concluded that the presence of clinical symptoms seemed to not be applicable to define PH. Furthermore, changes in calcium and PTH levels and their interdependence need to be considered to define PH.
Taking all these considerations into account, the following definition is suggested: PH can be defined as an undetectable or inappropriately low postoperative PTH level in the context of hypocalcaemia with or without hypocalcaemic symptoms. In this definition, the term ‘inappropriately low’ is thought to reflect the strong interdependence between PTH and calcium levels as even PTH in lower-normal ranges may be inappropriate to maintain normal calcium levels. Therefore, hypoparathyroidism may be present even when PTH levels seem to be in the normal range. As the development of symptoms is subjective, it remains unclear and not clearly quantifiable how low PTH levels can be to be considered as inappropriately low. It can be speculated whether the ratio between PTH and calcium levels is correlated with symptoms, and a prospective study specifically designed to address this question would be required.
Suitable time point to predict postsurgical hypoparathyroidism based on PTH levels
The significant correlation of reduced PTH levels with the manifestation of PH following thyroid surgery is well established. Many studies have aimed to determine a suitable time point for intraoperative or postoperative PTH measurements to predict the development of PH as early and precisely as possible.
The first aim was to determine whether intraoperative or postoperative PTH measurements are superior for early and specific prediction of developing PH. Intraoperative PTH measurements were usually carried out between 10 and 20 min after thyroidectomy or at the time point when surgery ended with skin closure.
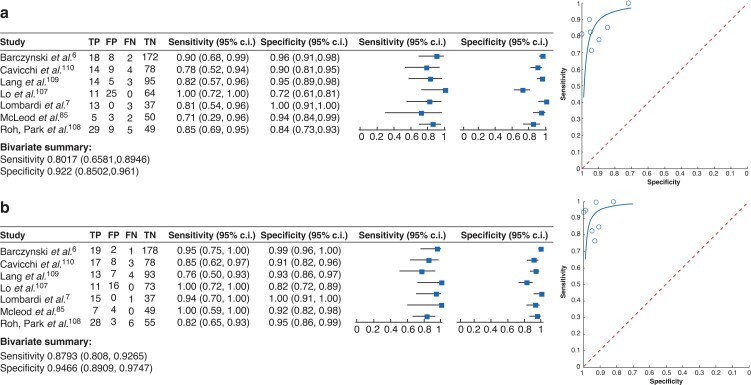
The direct comparison between intra- and postoperative PTH measurements was carried out in a total of 13 studies. Eight articles including 652 patients supported the view that postoperative PTH measurements are more sensitive and more specific compared with intraoperative PTH measurements in predicting PH7,22,83–85,105,106,176 (Table S3). Three articles including 392 patients did not show a significant difference between intra- and postoperative PTH measurements6,107,108 whereas two studies with 223 patients claimed that the intraoperative measurement of PTH is advantageous for early detection of PH109,110. Seven of these articles could be summarized in a meta-analysis (Fig. 2). The meta-analysis demonstrated a sensitivity of 80 per cent (95 per cent c.i. 0.66 to 0.90) and a specificity of 92 per cent (95 per cent c.i. 0.85 to 0.96) for intraoperative measurements of PTH values to be predictive for PH. However, with a sensitivity of 87 per cent (95 per cent c.i. 0.81 to 0.93) and a specificity of 95 per cent (95 per cent c.i. 0.89 to 0.98), the postoperative measurement of PTH levels seems to be superior compared with the intraoperative measurement of PTH levels to predict PH. Despite an overlap of confidence intervals, this seems to support the view of most studies that postsurgical measurements of PTH levels can be recommended for a reliable detection of PH rather than intraoperative measurements.
Fig. 2.
Forest plot (left) and summary receiver operating characteristic curves (right)
Studies analysing the value of a intraoperative parathyroid hormone measurements and b postoperative parathyroid hormone measurements to identify postsurgical hypoparathyroidism. The outcome parameter is hypocalcaemia in the presence or absence of symptoms as indicated by the study protocol of the studies included. Bivariate analysis summarized the sensitivity and specificity for each condition. TP, true positive; FP, false positive; FN, false negative; TN, true negative.
Next, the focus was on articles that performed postoperative PTH measurements with the aim of determining the best time point after thyroid surgery to detect PH. Overall, 21 articles were found to address the most suitable time point for PTH measurements ranging from 1 h and 6 h until 24 h after surgery or within the first postoperative day (POD1) (Table 2). All articles identified a time point of PTH measurement that was reported to be advantageous for early detection of PH, however none of the studies reported statistical significance when different time points were compared. In addition, there was extreme heterogeneity concerning study design, outcomes reported, and time points investigated. Therefore, only five studies were suitable to compare the sensitivity and specificity of two timeframes of postoperative PTH measurements, as they allowed direct comparison of the reported data (Fig. 3). It was decided to compare the timeframe within the first 6 and 24 h or within POD1 for PTH measurements after thyroid surgery as these timeframes were assessed most often in the literature and can result in clinical consequences. The early timeframe for measurements of PTH levels within 1–6 h after thyroid surgery resulted in an overall sensitivity of 88 per cent (95 per cent c.i. 0.81 to 0.92) and a specificity of 97 per cent (95 per cent c.i. 0.87 to 1.00) in predicting PH. The analysis of the later timeframe included data on post-surgical PTH measurements after 24 h or within POD1, which provided an overall sensitivity of 89 per cent (95 per cent c.i. 0.81 to 0.94) and a specificity of 98 per cent (95 per cent c.i. 0.86 to 1.00) in predicting PH. In summary, both timeframes resulted in almost equally high sensitivity and specificity values with small 95 per cent c.i. ranges in detecting PH. The conclusion therefore is that there is no distinct time after surgery that can be recommended for PTH measurements.
Table 2.
Overview of studies reporting the superiority of a defined time point for postoperative parathyroid hormone measurements
| Reference | Year of publication | Study design | Number of patients included | Time point(s) of PTH measurements | Statistical significance |
|---|---|---|---|---|---|
| Lam & Kerr42 | 2003 | Prospective | 40 | 1, 6 h | NA |
| Payne et al.a141,177 | 2003 | Prospective | 54 | 12 h | NA |
| Lombardi et al.7 | 2004 | Prospective | 53 | SC, 2, 4, 6, 24, 48 h | NA |
| Vescan et al.112 | 2005 | Prospective | 199 | 1 h, POD1 | NA |
| Payne et al.a 125 | 2005 | Prospective | 70 | 6h | NA |
| Roh & Park108 | 2006 | Prospective | 92 | SC,1 h, POD1 | NA |
| Sywak et al.48 | 2007 | Prospective | 100 | 4, 23 h | No difference |
| Al-Dhahri et al.132 | 2010 | Retrospective | 79 | 6, 12, 20, 32, 44h | No difference |
| J. H. Kim, Chung, & Son104 | 2011 | Retrospective | 112 | 1 h, POD1 | NA |
| Kim et al.83 | 2013 | Prospective | 108 | SC, 6, 12, 24, 48, 72 h | NA |
| Pisanu et al.55 | 2013 | Prospective | 112 | 6, 24, 48 h | NA |
| Al-Dhahri et al.164 | 2014 | Prospective | 168 | 1, 6 h | No difference |
| AlQahtani et al.32 | 2014 | NA | 149 | 1, 6, 24 h | No difference |
| Carr et al.90 | 2014 | Retrospective | 77 | 4 h, POD1 | No difference |
| Schlottmann et al.111 | 2015 | Prospective | 106 | 1, 3, 6 h | No difference |
| Sieniawski et al.20 | 2016 | Prospective | 142 | 1, 6 h | No difference |
| Yetkin et al.33 | 2016 | Prospective | 202 (SG) | 1, 24 h | No difference |
| +72 (CG) | |||||
| White et al.34 | 2016 | Prospective | 196 | 1 h, POD1 | No difference |
| Arer et al.96 | 2017 | Prospective | 106 | 6, 12, 24 h | No difference |
| Filho et al.36 | 2018 | Prospective | 101 | 1–4 h, POD1 | No difference |
Most of the studies described one superior time point for PTH measurements but none of them reported that the differences were statistically significant. PTH, parathyroid hormone; SC, PTH measurement skin closure; POD1, measurement postoperative day 1; NA, no data on statistically significant difference available: SG, study group; CG, control group as defined in the publication.
Studies can only be interpreted in combination, cited together.
Fig. 3.
Forest plot (left) and summary receiver operating characteristic curves (right)
Studies analysing a predictive value of early postoperative (1–6 h) and b later postoperative (24 h/POD1) parathyroid hormone measurements to identify postsurgical hypoparathyroidism. The definition of hypoparathyroidism varied between studies. Only studies were included in the meta-analysis that investigated both time points in the same cohort of patients. Bivariate analysis summarized the sensitivity and specificity for each condition. TP, true positive; FP, false positive; FN, false negative; TN, true negative.
It is reasonable that earlier measurements of PTH levels in the postoperative course will enable the early recognition of a potential problem and may lead to an indication for earlier therapeutic administration of calcium and vitamin D medication before patients develop symptoms. This is supported by 10 additional articles that could not be included in the meta-analysis showing the predictive value of PTH measurements 1 h after surgery is comparable to later time points20,32–34,42,104,108,111,112,164. This supports the main conclusion of the meta-analysis. The decision of which standard is the most applicable, however, will depend on the specific local facilities as PTH measurements may not be available outside core working hours.
Threshold levels for PTH to predict postsurgical hypoparathyroidism
The main problem in defining threshold levels for PTH is that there are different assays that result in different normal ranges of PTH levels178. This is supported by the observation that 81 studies defined 40 different PTH levels as the most reliable threshold to detect PH (Table S4). Therefore, to avoid assay-related confusion in preoperative PTH and calcium measurements, it is suggested that both PTH and calcium levels should be estimated by the same laboratories/institutions where postsurgical measurements will take place.
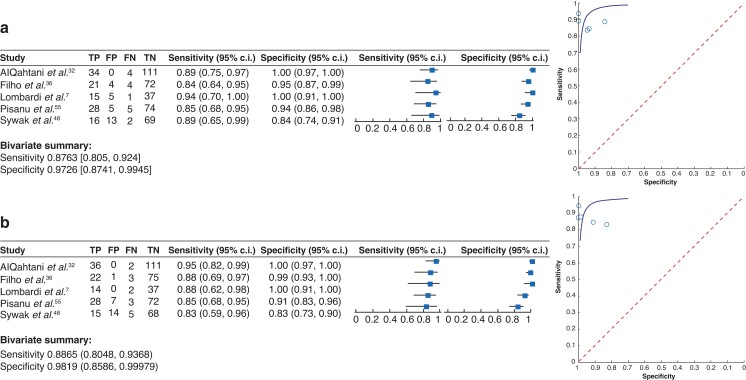
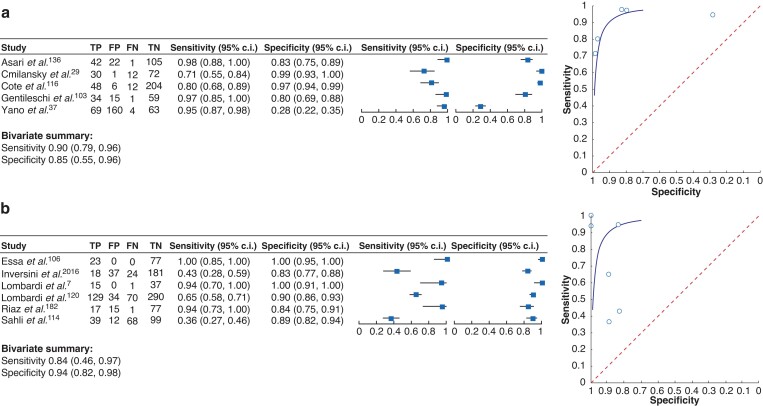
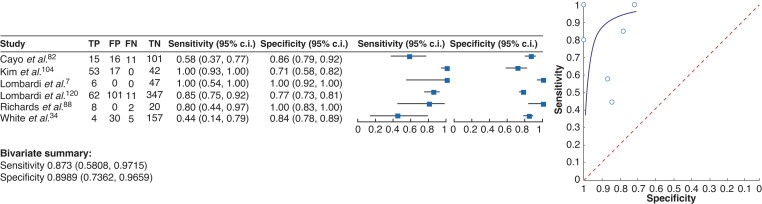
In addition, 20 articles were identified that analysed the diagnostic value of their lower limits of normal and included them in the more detailed analysis of the most commonly identified thresholds. In summary, 55 articles were eligible for further analysis. Seven articles that tested the predictive value of PTH levels below 20 pg/ml23,43,44,86,113–115, 19 articles on threshold levels below 15 pg/ml24,45–47,87,108,116–119 and 29 articles6,34,48,49,82,88,89,104,106,113,114,120–124,179,180 that tested the predictive value of PTH threshold levels below 10 pg/ml were identified. Out of these articles, the studies that aimed to identify PTH threshold levels as isolated parameters to predict PH were selected (Table 3). For each of the threshold levels of 10 pg/ml and 15 pg/ml, five studies were identified that analysed this aspect with a comparable study setting for further analyses (Fig. 4). The other studies could not be included because the study design was different, or data could not be extracted. It was not possible to summarize studies for the threshold level of 20 pg/ml due to their heterogeneity. When summarizing the results from the studies for 15 pg/ml (Fig. 4a) and 10 pg/ml (Fig. 4b), threshold levels of less than 15 pg/ml were found to have a sensitivity of 90 per cent (95 per cent c.i. 0.79 to 0.96) and a specificity of 85 per cent (95 per cent c.i. 0.55 to 0.96) in predicting PH. The threshold level of PTH values less than 10 pg/ml had a sensitivity of 84 per cent (95 per cent c.i. 0.46 to 0.97) and specificity of 94 per cent (95 per cent c.i. 0.82 to 0.98) in predicting PH. In an additional meta-analysis with studies that used threshold levels of 10 pg/ml to predict symptoms of hypoparathyroidism, a sensitivity of 87 per cent (95 per cent c.i. 0.58 to 0.97) and a specificity of 90 per cent (95 per cent c.i. 0.74 to 0.97) were found (Fig. 5). This led to the conclusion that both threshold levels are suitable to reliably predict the onset of PH. Taken together, using a threshold level that is oriented at the assay-specific lower limit of normal for PTH will lead to a high specificity and sensitivity for early detection of PH.
Table 3.
Overview of studies focusing on threshold levels of 10 pg/ml, 15 pg/ml and 20 pg/ml, including study design, number of patients, time point of parathyroid hormone measurement following thyroid surgery and endpoint to identify postsurgical hypoparathyroidism
| Threshold levels of PTH | Reference | Year of publication | Study design | Number of patients included | Lower limit of normal PTH (pg/ml) | Time point of PTH measurement | Endpoint |
|---|---|---|---|---|---|---|---|
| Threshold levels of PTH less than 10 pg/ml | Richards et al.88 | 2003 | Prospective | 30 | 12 pg/ml | SC | Symptoms |
| Lombardi et al.7 | 2004 | Prospective | 53 | 10 pg/ml | 4 h/6 h | Ca2+<2.0 mmol/l Symptoms | |
| Quiros et al.181 | 2005 | Prospective | 72 | 10 pg/ml | SC | Ca2+<2.1 mmol/l Symptoms | |
| Lombardi et al.120 | 2006 | Prospective | 523 | 10 pg/ml | 4 h | Ca2+<2.0 mmol/l Symptoms | |
| Barczyński et al.6 | 2007 | Prospective | 200 | 10 pg/ml | 4 h | Ca2+<2.0 mmol/l | |
| Gentileschi et al.103 | 2008 | Prospective | 119 | 15 pg/ml | 1 h | Symptoms | |
| Youngwirth et al.166 | 2010 | Retrospective | 371 | 10 pg/ml | 4 h/POD1 | Ca2+<2.1 mmol/l Symptoms | |
| Wiseman et al.46 | 2010 | Retrospective | 421 | 11 pg/ml | 1 h | Ca2+<2.2 mmol/l | |
| Ca2+<1.9 mmol/l | |||||||
| Kim et al.104 | 2011 | Retrospective | 112 | 11 pg/ml | 1 h | Symptoms | |
| Cayo et al.82 | 2012 | Prospective | 147 | Not reported | POD1 | Symptoms | |
| Riaz et al.182 | 2014 | Not reported | 110 | Not reported | 1 h | NA | |
| White et al.34 | 2016 | Prospective | 196 | 15 pg/ml | 1 h | Ca2+<2.0 mmol/l Symptoms | |
| Inversini et al.124 | 2016 | Retrospective | 260 | Not reported | 3–6 h | Ca2+<2.0 mmol/l Symptoms | |
| Al Khadem et al.122 | 2018 | Retrospective | 119 | 10 pg/ml | PACU | Ca2+<2 mmol/l Symptoms | |
| Sahli et al.114 | 2018 | Prospective | 218 | 10 pg/ml | 1 h | iCa2+<1.13 mmol/l Symptoms | |
| Essa et al.106 | 2021 | Prospective | 100 | 15 pg/ml | 10 min after TT | Ca2+<2.1 mmol/l Symptoms | |
| Abdullah et al.49 | 2021 | Retrospective | 57 | 10 pg/ml | 3 h | Ca2+<2.1 mmol/l | |
| Threshold levels of PTH less than 15 pg/ml | Warren et al.117 | 2002 | Retrospective | 53 | Not reported | IntraOp | iCa2+<1.0 mmol/l Symptoms |
| Chia et al.118 | 2006 | Prospective | 103 | Not reported | 8 h | Ca2+<1.9 mmol/l Symptoms | |
| Ghaheri et al.47 | 2006 | Retrospective | 80 | Not reported | PACU | iCa2+<1.0 mmol/l | |
| Chindavijak et al.58 | 2007 | Prospective | 30 | 15 pg/ml | IntraOp | Ca2+<2.1 mmol/l Symptoms | |
| Lewandowicz et al.17 | 2007 | Prospective | 54 | 15 pg/ml | SC | Ca2+< 2.1 mmol/l PTH | |
| Cote et al.116 | 2008 | Retrospective | 270 | Not reported | 1 h | Ca2+<2.0 mmol/l Symptoms | |
| Gentileschi et al.103 | 2008 | Prospective | 119 | 15 pg/ml | 1 h | Ca2+<2.0 mmol/l Symptoms | |
| Asari et al.136 | 2008 | Prospective | 170 | 15 pg/ml | POD1 | cCa2+<2.0 mmol/l Symptoms | |
| Huang et al.24 | 2012 | Prospective | 197 | 15 pg/ml | IntraOp | Ca2+<2.0 mmol/l | |
| Yano et al.37 | 2012 | Retrospective | 296 | 15 pg/ml | POD1 | cCa2+<2.0 mmol/l Symptoms | |
| Islam et al.45 | 2013 | Prospective | 65 | 12 pg/ml | IntraOp | Ca2+<2.0 mmol/l Symptoms | |
| Cmilansky et al.29 | 2014 | Prospective | 115 | 15 pg/ml | POD1 | Ca2+<2.0 mmol/l Symptoms | |
| Yetkin et al.33 | 2016 | Prospective | 274 | 15 pg/ml | 1 h | Ca2+<2.0 mmol/l Symptoms | |
| Threshold levels of PTH less than 20 pg/ml | Sabour et al.44 | 2009 | Retrospective | 448 | 15 pg/ml | PACU | cCa2+<2.0 mmol/l cCa2+<1.9 mmol/l |
| Proczko-Markuszewska et al.43 | 2010 | Prospective | 100 | 10 pg/ml | 1 h | Ca2+<2.0 mmol/l Symptoms | |
| Houlton et al.23 | 2011 | Retrospective | 180 | 15 pg/ml | PACU | Ca2+<2.0 mmol/l | |
| Noureldine et al.113 | 2014 | Retrospective | 304 | 10 pg/ml | 6–8 h | Symptoms Ca2+<2.0 mmol/l | |
| Lee et al.86 | 2015 | Prospective | 817 | Not reported | 1 h | Symptoms | |
| Sahli et al.114 | 2018 | Prospective | 218 | 10 pg/ml | 1 h | iCa2+<1.1 mmol/l Symptoms | |
| Bashir et al.115 | 2021 | Prospective | 175 (phase 1) | 14.9 pg/ml | Immediately after surgery | Ca2+<2.0 mmol/l Symptoms |
If required calcium and PTH values were adapted to pg/ml or mmol/l respectively. PTH, parathyroid hormone; POD1, postoperative day 1; IntraOp, measurement during surgery; cCa2+, corrected calcium; iCa2+, ionized calcium; PACU, post-anaesthesia care unit; NA, not available; SC, PTH measurement skin closure; TT, total thyroidectomy.
Fig. 4.
Forest plot (left) and summary receiver operating characteristic curves (right)
Studies of parathyroid hormone threshold levels of a less than 15 pg/ml and b less than 10 pg/ml to identify postsurgical hypoparathyroidism (development of hypocalcaemia). Bivariate analysis summarized the sensitivity and specificity for each condition. TP, true positive; FP, false positive; FN, false negative; TN, true negative.
Fig. 5.
Forest plot (left) and summary receiver operating characteristic curves (right)
Studies of parathyroid hormone threshold levels of less than10 pg/ml to identify postsurgical hypoparathyroidism (development of symptoms) are shown. Bivariate analysis summarized the sensitivity and specificity for each condition. TP, true positive; FP, false positive; FN, false negative; TN, true negative.
Relative reduction of pre- and postoperative PTH levels to predict postsurgical hypoparathyroidism
In view of the difficulties comparing different PTH assays it was proposed that a ratio between preoperative and postoperative PTH value may be suitable to reliably predict the manifestation of PH. In the literature, 51 articles were identified that focused on the relative reduction of PTH levels when pre- and postoperative measurements were compared. Looking at these articles systematically, 29 different ratios between preoperative and postoperative PTH values were found that were reported to predict PH. These ranged between a relative reduction of PTH preoperative/postoperative values of 19.4 per cent22 and 88.0 per cent183. Two studies sought to optimize the predictive value by forming risk groups in addition to the relative reduction of PTH levels after surgery, which resulted in the highest sensitivity and specificity to predict patients with PH122,164. This approach, however, seems to not be applicable in daily clinical practice. One of the main problems in determining a ratio between pre- and post-surgical PTH levels is that the time points of PTH measurements vary considerably in each study. As PTH levels show rapid changes under physiological conditions it can be impossible to exactly standardize time points of PTH measurements in daily routine. This is exemplified by a study that assessed PTH levels in 74 patients undergoing thyroidectomy before induction of anaesthesia, after induction of anaesthesia, 20 min after thyroidectomy and in the postoperative course184. This showed that during induction of anaesthesia, there is a relevant but unpredictable dynamic of PTH that changed to 149 (standard deviation 93) per cent of baseline levels (range 42–49.4 per cent) and normalized during surgery.
A meta-analysis by Noordzij et al. analysed nine studies to assess in more detail whether the relative loss of PTH before, during and after surgery can predict PH5. In 85 patients, a loss of more than 65 per cent of PTH levels compared before and 6 h after surgery had a sensitivity of 96.4 per cent and a specificity of 91.2 per cent to adequately predict PH.
In further analyses, eight original articles (Table 4) with comparable features in terms of pre- and postsurgical setting for PTH measurements were identified. Studies in which PTH measurements had been carried out before induction of anaesthesia were compared. When taking them together, a mean reduction of PTH levels of 73 ± 11 per cent was observed in the patient cohort that developed hypocalcaemia, whereas the group of patients with a mean reduction of PTH levels of 39.5 ± 7.3 per cent had no hypocalcaemia in the following course (P < 0.0001; Fig. 6).
Table 4.
Overview of the studies that tested the predictive value when the relative loss of parathyroid hormone levels between pre- and postoperative levels were compared
| Reference | Year of publication | Number of patients included | Mean postoperative reduction of PTH levels | |
|---|---|---|---|---|
| Patients without hypocalcaemia (%) | Patients with hypocalcaemia (%) | |||
| Roh/Park et al.108 | 2006 | 92 | 37 | 81 |
| Barczinsky et al.6 | 2007 | 200 | 32 | 69 |
| Toniato et al.69 | 2008 | 160 | 40 | 63 |
| Mehrvarz et al.127 | 2014 | 99 | 41 | 60 |
| Puzziello et al.56 | 2015 | 75 | 44 | 62 |
| Seo et al.59 | 2015 | 349 | 49 | 80 |
| Sieniawski et al.20 | 2016 | 142 | 36 | 82 |
| Suwannasarn et al.54 | 2017 | 65 | 29 | 83 |
| Mo et al.170 | 2020 | 176 | 53 | 86 |
| Mean(s.d.) | 1358 | 39.5(7.3) | 73.0(11) | |
PTH, parathyroid hormone.
Fig. 6.
Median of mean and 95 per cent confidence intervals from values of relative reduction of parathyroid hormone levels from eight comparable studies
These were extracted to assess whether these values can be used to predict hypocalcaemia in patients after thyroid surgery. All studies together represent a cohort of 1358 patients. Unpaired non-parametric Kruskal–Wallis test was used to test for significant differences. PTH, parathyroid hormone.
Based on this and on the results of the meta-analysis described above, it can be concluded that a relative reduction of PTH of more than 70 per cent after surgery can be predictive for the development of PH. On the other hand, this should be considered with caution as, in addition to the measurement uncertainty, other physiological factors, including vitamin D status may affect the relative loss of PTH levels after surgery. The relationship between preoperative vitamin D status and the development of PH is controversial as there are a number of manuscripts supporting this185–190, and some that do not show a significant relationship35,191–199. Due to this, all studies independent of the vitamin D status were included in this meta-analysis.
Role of postsurgical calcium measurements
It goes without saying, that PTH measurements do not replace the need to control postsurgical calcium levels. Therefore, it is broadly accepted and recommended that calcium measurements should be carried out after surgery at least on POD1, whereas some favour including measurements on POD29,200. In cases, in which PTH levels have been measured at appropriate levels after surgery and calcium levels on POD1 are in the normal range, it may be discussed that the control of calcium levels on POD2 are dispensable. This is supported by three articles confirming that the combination of early PTH measurements and calcium estimation on POD1 is a safe procedure for patients to reliably identify those patients who will not develop PH123,125,126.
In summary, based on the present review of the literature, structured surveillance of perioperative parathyroid function in thyroid surgery is recommended, which should include early postsurgical PTH values to decide on (prophylactic) therapeutic intervention that should be completed by estimation of calcium levels at least on POD1. In cases of abnormal results such as low levels of calcium and PTH or hypocalcaemic symptoms, measurements of calcium should be repeated on POD2.
Discussion
The literature provides a lot of heterogeneous and observational studies focusing on the problem of PH. In the future, a consensus-based uniform definition for PH should be developed to provide the basis for future studies and clinical application. Based on this review and meta-analysis and keeping the mentioned limitations in mind, the key conclusions and suggestions are as follows:
PH can be defined as an undetectable or inappropriately low postoperative PTH level in the context of hypocalcaemia with or without hypocalcaemic symptoms.
Postsurgical measurements of PTH levels have a higher sensitivity and specificity than intraoperative PTH measurements in predicting PH.
The ideal time point of the measurements of postsurgical PTH levels to predict PH is between the end of the operation until 24 h after surgery. There are no significant differences within this timeframe.
Serum PTH levels as a threshold for detecting PH most often corresponded to the lower levels of normal of laboratories where PTH measurements were carried out. According to the meta-analysis, PTH levels below 15 and 10 pg/ml give a high sensitivity and specificity in predicting the development of PH. Using a threshold level that is oriented at the assay-specific lower limit of normal for PTH for early detection of PH is suggested.
A PTH decrease from pre- to postoperative sampling of more than 73 ± 11 per cent seems to predict the development of PH, provided that the preoperative measurements are carried out in the same laboratory and before induction of anaesthesia. In addition to the measurement uncertainty and other physiological factors including the vitamin D status, the reliability of the relative reduction of PTH should be used with caution.
Independent from PTH measurements, the estimation of calcium levels on POD1 should be carried out. Additional calcium measurements may not be required if PTH and calcium values are normal in the early postoperative course and patients do not develop symptoms201–203.
Supplementary Material
Contributor Information
Kathrin Nagel, Department of General, Visceral, Transplant, Vascular and Pediatric Surgery, University Hospital Würzburg, Würzburg, Germany.
Anne Hendricks, Department of General, Visceral, Transplant, Vascular and Pediatric Surgery, University Hospital Würzburg, Würzburg, Germany.
Christina Lenschow, Department of General, Visceral, Transplant, Vascular and Pediatric Surgery, University Hospital Würzburg, Würzburg, Germany.
Michael Meir, Department of General, Visceral, Transplant, Vascular and Pediatric Surgery, University Hospital Würzburg, Würzburg, Germany.
Stefanie Hahner, Department of Internal Medicine, Division of Endocrinology and Diabetes, University Hospital, University of Würzburg, Würzburg, Germany.
Martin Fassnacht, Department of Internal Medicine, Division of Endocrinology and Diabetes, University Hospital, University of Würzburg, Würzburg, Germany.
Armin Wiegering, Department of General, Visceral, Transplant, Vascular and Pediatric Surgery, University Hospital Würzburg, Würzburg, Germany.
Christoph-Thomas Germer, Department of General, Visceral, Transplant, Vascular and Pediatric Surgery, University Hospital Würzburg, Würzburg, Germany.
Nicolas Schlegel, Department of General, Visceral, Transplant, Vascular and Pediatric Surgery, University Hospital Würzburg, Würzburg, Germany.
Funding
The authors have no funding to declare.
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability statement
The data of this review and meta-analysis can be made available to any researcher. All relevant data are included in the tables and supplemental material. All other material and data can be provided directly on request to the corresponding author.
References
- 1. Edafe O, Antakia R, Laskar N, Uttley L, Balasubramanian SP. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg 2014;101:307–320 [DOI] [PubMed] [Google Scholar]
- 2. Orloff LA, Wiseman SM, Bernet VJ, Fahey TJ III, Shaha AR, Shindo ML et al. American thyroid association statement on postoperative hypoparathyroidism: diagnosis, prevention, and management in adults. Thyroid 2018;28:830–841 [DOI] [PubMed] [Google Scholar]
- 3. Qiu Y, Xing Z, Fei Y, Qian Y, Luo Y, Su A. Role of the 2018 American Thyroid Association statement on postoperative hypoparathyroidism: a 5-year retrospective study. BMC Surg 2021;21:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sitges-Serra A. Etiology and diagnosis of permanent hypoparathyroidism after total thyroidectomy. J Clin Med 2021;10:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Noordzij JP, Lee SL, Bernet VJ, Payne RJ, Cohen SM, McLeod IK et al. Early prediction of hypocalcemia after thyroidectomy using parathyroid hormone: an analysis of pooled individual patient data from nine observational studies. J Am Coll Surg 2007;205:748–754 [DOI] [PubMed] [Google Scholar]
- 6. Barczynski M, Cichon S, Konturek A. Which criterion of intraoperative iPTH assay is the most accurate in prediction of true serum calcium levels after thyroid surgery? Langenbecks Arch Surg 2007;392:693–698 [DOI] [PubMed] [Google Scholar]
- 7. Lombardi CP, Raffaelli M, Princi P, Santini S, Boscherini M, De Crea C et al. Early prediction of postthyroidectomy hypocalcemia by one single iPTH measurement. Surgery 2004;136:1236–1241 [DOI] [PubMed] [Google Scholar]
- 8. AES Guidelines 06/01 Group. Australian Endocrine Surgeons Guidelines AES06/01 . Postoperative parathyroid hormone measurement and early discharge after total thyroidectomy: analysis of Australian data and management recommendations. ANZ J Surg 2007;77:199–202 [DOI] [PubMed] [Google Scholar]
- 9. Hosseini M, Otaghvar HA, Tizmaghz A, Shabestanipour G, Vahid PA. Evaluating the time interval for presenting the signs of hypocalcaemia after thyroidectomy. J Clin Diagn Res 2016;10:PC19–PC22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Del Rio P, Arcuri MF, Ferreri G, Sommaruga L, Sianesi M. The utility of serum PTH assessment 24 h after total thyroidectomy. Otolaryngol Head Neck Surg 2005;132:584–586 [DOI] [PubMed] [Google Scholar]
- 11. Lee YS, Chang HS, Chung WY, Nam KH, Park CS. Relationship between onset of hypocalcemic symptoms and the recovery time from transient hypocalcemia after total thyroidectomy. Head Neck 2014;36:1732–1736 [DOI] [PubMed] [Google Scholar]
- 12. Huang R, Wang Q, Zhang W, Zha S, Jiang D, Xu X et al. The predictive factors for postoperative hypoparathyroidism and its severity on the first postoperative day after papillary thyroid carcinoma surgery. Eur Arch oto-rhino-laryngol 2020;278:1189–1198. [DOI] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stern C, Jordan Z, McArthur A. Developing the review question and inclusion criteria. AJN Am J Nurs 2014;114:53–56 [DOI] [PubMed] [Google Scholar]
- 15. Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Software 2012;49:1–15 [Google Scholar]
- 17. Lewandowicz M, Kuzdak K, Pasieka Z. Intraoperative parathyroid hormone measurement in thyroidectomized patients: preliminary report. Endocr Regul 2007;41:29–34 [PubMed] [Google Scholar]
- 18. Ezzat WF, Fathey H, Fawaz S, El-Ashri A, Youssef T, Othman HB. Intraoperative parathyroid hormone as an indicator for parathyroid gland preservation in thyroid surgery. Swiss Med wkly 2011;141:w13299. [DOI] [PubMed] [Google Scholar]
- 19. Yazıcıoğlu M, Yılmaz A, Kocaöz S, Özçağlayan R, Parlak Ö. Risks and prediction of postoperative hypoparathyroidism due to thyroid surgery. Sci Rep 2021;11:11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sieniawski K, Kaczka K, Paduszynska K, Fendler W, Tomasik B, Pomorski L. Early predictors of post-thyroidectomy hypoparathyroidism. Pol Przegl Chir 2016;88:305–314 [DOI] [PubMed] [Google Scholar]
- 21. Cho JN, Park WS, Min SY. Predictors and risk factors of hypoparathyroidism after total thyroidectomy. Int J Surg 2016;34:47–52 [DOI] [PubMed] [Google Scholar]
- 22. Melo F, Bernardes A, Velez A, Campos de Melo C, de Oliveira FJ. Parathyroid hormone as a predictor of post-thyroidectomy hypocalcemia: a prospective evaluation of 100 patients. Acta Med Port 2015;28:322–328 [PubMed] [Google Scholar]
- 23. Houlton JJ, Pechter W, Steward DL. PACU PTH facilitates safe outpatient total thyroidectomy. Otolaryngol Head Neck Surg 2011;144:43–47 [DOI] [PubMed] [Google Scholar]
- 24. Huang SM. Do we overtreat post-thyroidectomy hypocalcemia? World J Surg 2012;36:1503–1508 [DOI] [PubMed] [Google Scholar]
- 25. Rosa KM, Matos LL, Cernea CR, Brandao LG, Araujo Filho VJ. Postoperative calcium levels as a diagnostic measure for hypoparathyroidism after total thyroidectomy. Arch Endocrinol Metab 2015;59:428–433 [DOI] [PubMed] [Google Scholar]
- 26. Raffaelli M, De Crea C, D’Amato G, Moscato U, Bellantone C, Carrozza C et al. Post-thyroidectomy hypocalcemia is related to parathyroid dysfunction even in patients with normal parathyroid hormone concentrations early after surgery. Surgery 2016;159:78–85 [DOI] [PubMed] [Google Scholar]
- 27. Selberherr A CS, Riss P, Niederle B. Postoperative hypoparathyroidism after thyroidectomy: efficient and cost-effective diagnosis and treatment. Surgery 2015;157:349–353 [DOI] [PubMed] [Google Scholar]
- 28. Gupta S, Chaudhary P, Durga CK, Naskar D. Validation of intra-operative parathyroid hormone and its decline as early predictors of hypoparathyroidism after total thyroidectomy: a prospective cohort study. Int J Surg 2015;18:150–153 [DOI] [PubMed] [Google Scholar]
- 29. Cmilansky P, Mrozova L. Hypocalcemia - the most common complication after total thyroidectomy. Bratisl Lek Listy 2014;115:175–178 [DOI] [PubMed] [Google Scholar]
- 30. Marcinkowska M, Sniecikowska B, Zygmunt A, Brzezinski J, Dedecjus M, Lewinski A. Postoperative hypoparathyroidism in patients after total thyroidectomy - retrospective analysis. Neuro Endocrinol Lett 2017;38:488–494 [PubMed] [Google Scholar]
- 31. Landry CS, Grubbs EG, Hernandez M, Hu MI, Hansen MO, Lee JE et al. Predictable criteria for selective, rather than routine, calcium supplementation following thyroidectomy. Arch Surg 2012;147:338–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. AlQahtani A, Parsyan A, Payne R, Tabah R. Parathyroid hormone levels 1 h after thyroidectomy: an early predictor of postoperative hypocalcemia. Can J Surg 2014;57:237–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yetkin G, Citgez B, Yazici P, Mihmanli M, Sit E, Uludag M. Early prediction of post-thyroidectomy hypocalcemia by early parathyroid hormone measurement. Ann Ital Chir 2016;87:417–421 [PubMed] [Google Scholar]
- 34. White MG, James BC, Nocon C, Nagar S, Kaplan EL, Angelos P et al. One-hour PTH after thyroidectomy predicts symptomatic hypocalcemia. J Surg Res 2016;201:473–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Manzini G, Malhofer F, Weber T. Can preoperative vitamin D deficiency predict postoperative hypoparathyroidism following thyroid surgery? Langenbecks Arch Surg 2019;404:55–61 [DOI] [PubMed] [Google Scholar]
- 36. Filho EBY, Machry RV, Mesquita R, Scheffel RS, Maia AL. The timing of parathyroid hormone measurement defines the cut-off values to accurately predict postoperative hypocalcemia: a prospective study. Endocrine 2018;61:224–231 [DOI] [PubMed] [Google Scholar]
- 37. Yano Y, Masaki C, Sugino K, Nagahama M, Kitagawa W, Sibuya H et al. Serum intact parathyroid hormone level after total thyroidectomy or total thyroidectomy plus lymph node dissection for thyroid nodules: report from 296 surgical cases. Int J Endocrinol Metab 2012;10:594–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sands N, Young J, MacNamara E, Black MJ, Tamilia M, Hier MP et al. Preoperative parathyroid hormone levels as a predictor of postthyroidectomy hypocalcemia. Otolaryngol Head Neck Surg 2011;144:518–521 [DOI] [PubMed] [Google Scholar]
- 39. De Pasquale L, Sartori PV, Vicentini L, Beretta E, Boniardi M, Leopaldi E et al. Necessity of therapy for post-thyroidectomy hypocalcaemia: a multi-centre experience. Langenbecks Arch Surg 2015;400:319–324 [DOI] [PubMed] [Google Scholar]
- 40. Salinger EM, Moore JT. Perioperative indicators of hypocalcemia in total thyroidectomy: the role of vitamin D and parathyroid hormone. Am J Surg 2013;206:876–882 [DOI] [PubMed] [Google Scholar]
- 41. Palmhag D, Brydolf J, Zedenius J, Branstrom R, Nilsson IL. A single parathyroid hormone measurement two hours after a thyroidectomy reliably predicts permanent hypoparathyroidism. Scand J Surg 2021;110:322––328. [DOI] [PubMed] [Google Scholar]
- 42. Lam A, Kerr PD. Parathyroid hormone: an early predictor of postthyroidectomy hypocalcemia. The Laryngoscope 2003;113:2196–2200 [DOI] [PubMed] [Google Scholar]
- 43. Proczko-Markuszewska M, Kobiela J, Stefaniak T, Lachinski AJ, Sledzinski Z. Postoperative PTH measurement as a predictor of hypocalcaemia after thyroidectomy. Acta Chir Belg 2010;110:40–44 [DOI] [PubMed] [Google Scholar]
- 44. Sabour S, Manders E, Steward DL. The role of rapid PACU parathyroid hormone in reducing post-thyroidectomy hypocalcemia. Otolaryngol Head Neck Surg 2009;141:727–729 [DOI] [PubMed] [Google Scholar]
- 45. Islam MS, Sultana T, Paul D, Huq AHMZ, Chowdhury AA, Ferdous C et al. Intraoperative serum parathyroid hormone level is an indicator of hypocalcaemia in total thyroidectomy patients. Bangladesh Med Res Counc Bull 2013;38:84–89 [DOI] [PubMed] [Google Scholar]
- 46. Wiseman JE, Mossanen M, Ituarte PH, Bath JM, Yeh MW. An algorithm informed by the parathyroid hormone level reduces hypocalcemic complications of thyroidectomy. World J Surg 2010;34:532–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ghaheri BA, Liebler SL, Andersen PE, Schuff KG, Samuels MH, Klein RF et al. Perioperative parathyroid hormone levels in thyroid surgery. Laryngoscope 2006;116:518–521 [DOI] [PubMed] [Google Scholar]
- 48. Sywak MS, Palazzo FF, Yeh M, Wilkinson M, Snook K, Sidhu SB et al. Parathyroid hormone assay predicts hypocalcaemia after total thyroidectomy. ANZ J Surg 2007;77:667–670 [DOI] [PubMed] [Google Scholar]
- 49. Abdullah AS. The role of early postoperative parathyroid hormone level after total thyroidectomy in prediction of hypocalcemia. Ann Med Surg 2021;65:102252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tartaglia F, Giuliani A, Sgueglia M, Patrizi G, Di Rocco G, Blasi S et al. Is ionized calcium a reliable predictor of hypocalcemia after total thyroidectomy? A before and after study. G Chir 2014;35:27–35 [PMC free article] [PubMed] [Google Scholar]
- 51. Tredici P, Grosso E, Gibelli B, Massaro MA, Arrigoni C, Tradati N. Identification of patients at high risk for hypocalcemia after total thyroidectomy. Acta Otorhinolaryngol Ital 2011;31:144–148 [PMC free article] [PubMed] [Google Scholar]
- 52. Sousa A dA, Salles JMP, Soares JMA, de Moraes GM, Carvalho JR, Rocha PRS. Course of ionized calcium after thyroidectomy. World J Surg 2010;34:987–992 [DOI] [PubMed] [Google Scholar]
- 53. Chow TL, Choi CY, Chiu ANK. Postoperative PTH monitoring of hypocalcemia expedites discharge after thyroidectomy. Am J Otolaryngol 2014;35:736–740 [DOI] [PubMed] [Google Scholar]
- 54. Suwannasarn M, Jongjaroenprasert W, Chayangsu P, Suvikapakornkul R, Sriphrapradang C. Single measurement of intact parathyroid hormone after thyroidectomy can predict transient and permanent hypoparathyroidism: a prospective study. Asian J Surg 2017;40:350–356 [DOI] [PubMed] [Google Scholar]
- 55. Pisanu A, Saba A, Coghe F, Uccheddu A. Early prediction of hypocalcemia following total thyroidectomy using combined intact parathyroid hormone and serum calcium measurement. Langenbecks Arch Surg 2013;398:423–430 [DOI] [PubMed] [Google Scholar]
- 56. Puzziello A, Gervasi R, Orlando G, Innaro N, Vitale M, Sacco R. Hypocalcaemia after total thyroidectomy: could intact parathyroid hormone be a predictive factor for transient postoperative hypocalcemia? Surgery 2015;157:344–348 [DOI] [PubMed] [Google Scholar]
- 57. Alia P, Moreno P, Rigo R, Francos JM, Navarro MA. Postresection parathyroid hormone and parathyroid hormone decline accurately predict hypocalcemia after thyroidectomy. Am J Clin Pathol 2007;127:592–597 [DOI] [PubMed] [Google Scholar]
- 58. Chindavijak S. Prediction of hypocalcemia in postoperative total thyroidectomy using single measurement of intra-operative parathyroid hormone level. J Med Assoc Thai 2007;90:1167–1171 [PubMed] [Google Scholar]
- 59. Seo ST, Chang JW, Jin J, Lim YC, Rha KS, Koo BS. Transient and permanent hypocalcemia after total thyroidectomy: Early predictive factors and long-term follow-up results. Surgery 2015;158:1492–1499 [DOI] [PubMed] [Google Scholar]
- 60. Cannizzaro MA, Okatyeva V, Lo Bianco S, Caruso V, Buffone A. Hypocalcemia after thyroidectomy: iPTH levels and iPTH decline are predictive? Retrospective cohort study. Ann Med Surg 2018;30:42–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cherian AJ, Ramakant P, Paul TV, Abraham DT, Paul MJ. Next-day parathyroid hormone as a predictor of post-thyroidectomy hypocalcemia. World J Endocr Surg 2016;8:203–207 [Google Scholar]
- 62. Cahill RA, Harty R, Cotter S, Watson RGK. Parathormone response to thyroid surgery. Am J Surg 2006;191:453–459 [DOI] [PubMed] [Google Scholar]
- 63. Košec A, Hergešić F, Matovinović F, Rašić I, Vagić D, Bedeković V. Identifying early postoperative serum parathyroid hormone levels as predictors of hypocalcaemia after total thyroidectomy: a prospective non-randomized study. Am J Otolaryngol 2020;41:102416. [DOI] [PubMed] [Google Scholar]
- 64. Strajina V, Dy BM, McKenzie TJ, Thompson GB, Lyden ML. Predicting postthyroidectomy hypocalcemia: improving predictive ability of parathyroid hormone level. Am Surg 2020;86:121–126 [PubMed] [Google Scholar]
- 65. Kakava K, Tournis S, Makris K, Papadakis G, Kassi E, Dontas I et al. Identification of patients at high risk for postsurgical hypoparathyroidism. In vivo 2020;34:2973–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Karatzanis AD, Ierodiakonou DP, Fountakis ES, Velegrakis SG, Doulaptsi MV, Prokopakis EP et al. Postoperative day 1 levels of parathyroid as predictor of occurrence and severity of hypocalcaemia after total thyroidectomy. Head Neck 2018;40:1040–1045 [DOI] [PubMed] [Google Scholar]
- 67. Grodski S, Farrell S. Early postoperative PTH levels as a predictor of hypocalcaemia and facilitating safe early discharge after total thyroidectomy. Asian J Surg 2007;30:178–182 [DOI] [PubMed] [Google Scholar]
- 68. Graff AT, Miller FR, Roehm CE, Prihoda TJ. Predicting hypocalcemia after total thyroidectomy: parathyroid hormone level vs. serial calcium levels. Ear Nose Throat J 2010;89:462–465 [PubMed] [Google Scholar]
- 69. Toniato A, Boschin IM, Piotto A, Pelizzo M, Sartori P. Thyroidectomy and parathyroid hormone: tracing hypocalcemia-prone patients. Am J Surg 2008;196:285–288 [DOI] [PubMed] [Google Scholar]
- 70. Walsh SR, Kumar B, Coveney EC. Serum calcium slope predicts hypocalcaemia following thyroid surgery. Int J Surg 2007;5:41–44 [DOI] [PubMed] [Google Scholar]
- 71. Costanzo M, Marziani A, Condorelli F, Migliore M, Cannizzaro MA. Post-thyroidectomy hypocalcemic syndrome: predictive value of early PTH. Preliminary results. Ann Ital Chir 2010;81:301–305 [PubMed] [Google Scholar]
- 72. Bozec A, Guevara N, Bailleux S, Castillo L, Santini J. Early PTH assay after total thyroidectomy: predictive factor for postoperative hypocalcemia? Rev Laryngol Otol Rhinol 2006;127:141–144 [PubMed] [Google Scholar]
- 73. Saba A, Podda M, Messina Campanella A, Pisanu A. Early prediction of hypocalcemia following thyroid surgery. a prospective randomized clinical trial. Langenbecks Arch Surg 2017;402:1119–1125 [DOI] [PubMed] [Google Scholar]
- 74. Bove A, Di Renzo RM, Palone G, D’Addetta V, Percario R, Panaccio P et al. Early biomarkers of hypocalcemia following total thyroidectomy. Int J Surg 2014;12:S202–204 [DOI] [PubMed] [Google Scholar]
- 75. Flores-Pastor B, Miquel-Perello J, Del Pozo P, Perez A, Soria-Aledo V, Aguayo-Albasini JL. [Diagnostic value of intraoperative parathyroid hormone decline in prediction of hypocalcemia after total thyroidectomy]. Med Clin 2009;132:136–139 [DOI] [PubMed] [Google Scholar]
- 76. O’Neill CJ, Jinih M, Boyle S, Brennan SA, Majeed M, Achakzai AA et al. Risk reduction of hypocalcemia after thyroidectomy: review of a clinical practice in an Irish cohort. Eur Surg Acta Chir Austriaca 2018;50:8–13 [Google Scholar]
- 77. Luu Q, Andersen PE, Adams J, Wax MK, Cohen JI. The predictive value of perioperative calcium levels after thyroid/parathyroid surgery. Head Neck 2002;24:63–67 [DOI] [PubMed] [Google Scholar]
- 78. Adams J, Andersen P, Everts E, Cohen J. Early postoperative calcium levels as predictors of hypocalcemia. The Laryngoscope 1998;108:1829–1831 [DOI] [PubMed] [Google Scholar]
- 79. Stedman T, Truran P, Harrison B, Balasubramanian S. Postoperative hypocalcaemia after bilateral thyroid surgery. Closed loop audit. Int J Surg 2016;36:S72 [Google Scholar]
- 80. Kim DS, Barber AE, Wang R. Early prediction of post-thyroidectomy hypocalcemia using intraoperative parathyroid hormone assay. Thyroid 2015;25:71–7725285888 [Google Scholar]
- 81. Kahan S, Najafian A, Mathur A, Schneider EB, Zeiger M. Lowparathyroidhormonelevels actuallydo not predict the need for calcium supplementation after total thyroidectomy. Thyroid 2015;25:A173–A174 [Google Scholar]
- 82. Cayo AK, Yen TW, Misustin SM, Wall K, Wilson SD, Evans DB et al. Predicting the need for calcium and calcitriol supplementation after total thyroidectomy: results of a prospective, randomized study. Surgery 2012;152:1059–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kim JP, Park JJ, Son HY, Kim RB, Kim HY, Woo SH. Effectiveness of an i-PTH measurement in predicting post thyroidectomy hypocalcemia: prospective controlled study. Yonsei Med J 2013;54:637–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hermann M, Ott J, Promberger R, Kober F, Karik M, Freissmuth M. Kinetics of serum parathyroid hormone during and after thyroid surgery. Br J Surg 2008;95:1480–1487 [DOI] [PubMed] [Google Scholar]
- 85. McLeod IK, Arciero C, Noordzij JP, Stojadinovic A, Peoples G, Melder PC et al. The use of rapid parathyroid hormone assay in predicting postoperative hypocalcemia after total or completion thyroidectomy. Thyroid 2006;16:259–265 [DOI] [PubMed] [Google Scholar]
- 86. Lee YM, Cho JY, Sung TY, Kim TY, Chung KW, Hong SJ et al. Clinicopathological risk factors and biochemical predictors of safe discharge after total thyroidectomy and central compartment node dissection for thyroid cancer: a prospective study. Int J Endocrinol 2015;2015:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. An CM, Tang PZ, Xu ZG, Zhang B, Zhang ZM, Yan DG et al. Role of parathyroid hormone measurement in prediction for symptomatic hypocalcaemia after total thyroidectomy. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2010;45:217–221 [PubMed] [Google Scholar]
- 88. Richards ML, Bingener-Casey J, Pierce D, Strodel WE, Sirinek KR. Intraoperative parathyroid hormone assay: an accurate predictor of symptomatic hypocalcemia following thyroidectomy. Arch Surg (Chicago, Ill: 1960) 2003;138:632–635 [DOI] [PubMed] [Google Scholar]
- 89. Carter Y, Chen H, Sippel RS. An intact parathyroid hormone-based protocol for the prevention and treatment of symptomatic hypocalcemia after thyroidectomy. J Surg Res 2013;186:23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Carr AA, Yen TW, Fareau GG, Cayo AK, Misustin SM, Evans DB et al. A single parathyroid hormone level obtained 4 h after total thyroidectomy predicts the need for postoperative calcium supplementation. J Am Coll Surg 2014;219:757–764 [DOI] [PubMed] [Google Scholar]
- 91. Sebastian M, Rudnicki J, Jakubaszko W, Zyśko D, Agrawal AK, Sebastian A. Clinical and biochemical factors affecting postoperative hypocalcemia after near-total thyroidectomy. Adv Clin Exp Med 2013;87:675–682 [PubMed] [Google Scholar]
- 92. Wong C, Price S, Scott-Coombes D. Hypocalcaemia and parathyroid hormone assay following total thyroidectomy: predicting the future. World J Surg 2006;30:825–832 [DOI] [PubMed] [Google Scholar]
- 93. Vanderlei FA, Vieira JG, Hojaij FC, Cervantes O, Kunii IS, Ohe MN et al. Parathyroid hormone: an early predictor of symptomatic hypocalcemia after total thyroidectomy. Arq Bras Endocrinol Metabol 2012;56:168–172 [DOI] [PubMed] [Google Scholar]
- 94. Zhou TJ, Zhang JC, Lu W, Zhao F, Li XF, Chen B. The predictive value of parathyroid hormone levels and decreases for postoperative hypocalcemia after total thyroidectomy. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2017;31:1880–1883 [DOI] [PubMed] [Google Scholar]
- 95. Castro A, Del Rio L, Gavilan J. Stratifying the risk of developing clinical hypocalcemia after thyroidectomy with parathyroid hormone. Otolaryngol Head Neck Surg 2018;158:76–82 [DOI] [PubMed] [Google Scholar]
- 96. Arer IM, Kus M, Akkapulu N, Aytac HO, Yabanoglu H, Caliskan K et al. Prophylactic oral calcium supplementation therapy to prevent early post thyroidectomy hypocalcemia and evaluation of postoperative parathyroid hormone levels to detect hypocalcemia: a prospective randomized study. Int J Surg 2017;38:9–14 [DOI] [PubMed] [Google Scholar]
- 97. Luo H, Yang H, Wei T, Gong Y, Su A, Ma Y et al. Protocol for management after thyroidectomy: a retrospective study based on one-center experience. Ther Clin Risk Manag 2017;13:635–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gutierrez G, Garcia J, Toledo E, Del Castillo A, Cañon M, Casanova D. Short stay thyroidectomy based in quick parathyroid hormone determination. Langenbecks Arch Surg 2017;402:398 [Google Scholar]
- 99. Goh S, Rao A, Singaporewalla R. Use of serum parathyroid hormone (PTH) and ionized calcium (ICA) trend as a predictor of early next day discharge after total thyroidectomy. Thyroid 2017;27:A96 [Google Scholar]
- 100. Yun N, Lee Y, Cho J, Sung T, Chung K, Hong S et al. Predictors of the development of hypocalcemic symptoms according to postoperative days after total thyroidectomy. Thyroid 2013;23:A59 [Google Scholar]
- 101. Scurry WC Jr, Beus KS, Hollenbeak CS, Stack BC Jr. Perioperative parathyroid hormone assay for diagnosis and management of postthyroidectomy hypocalcemia. Laryngoscope 2005; 115:1362–1366. [DOI] [PubMed] [Google Scholar]
- 102. Bahler S, Muller W, Linder T, Frotzler A, Fischli S, Aqtashi B et al. Intraoperative parathyroid hormone measurement is the best predictor of postoperative symptomatic hypocalcemia. Hno 2017;65:1000–1007 [DOI] [PubMed] [Google Scholar]
- 103. Gentileschi P, Gacek IA, Manzelli A, Coscarella G, Sileri P, Lirosi F et al. Early (1 h) post-operative parathyroid hormone (PTH) measurement predicts hypocalcaemia after thyroidectomy: a prospective case-control single-institution study. Chir Italiana 2008;60:519–528 [PubMed] [Google Scholar]
- 104. Kim JH, Chung MK, Son YI. Reliable early prediction for different types of post-thyroidectomy hypocalcemia. Clin Exp Otorhinolaryngol 2011;4:95–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Del Río L, Castro A, Bernáldez R, Del Palacio A, Giráldez CV, Lecumberri B et al. Parathyroid hormone as a predictor of post-thyroidectomy hypocalcemia. Acta Otorrinolaringol Esp 2011;62:256–273 [DOI] [PubMed] [Google Scholar]
- 106. Essa MS, Ahmad KS, Fadey MA, El-Shaer MO, Salama AMF, Zayed ME. Role of perioperative parathormone hormone level assay after total thyroidectomy as a predictor of transient and permanent hypocalcemia: prospective study. Ann Med Surg 2021;69:102701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lo CY, Luk JM, Tam SC. Applicability of intraoperative parathyroid hormone assay during thyroidectomy. Ann Surg 2002;236:564–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Roh JL, Park CIL. Intraoperative parathyroid hormone assay for management of patients undergoing total thyroidectomy. Head Neck 2006;28:990–997 [DOI] [PubMed] [Google Scholar]
- 109. Lang BH, Yih PC, Ng KK. A prospective evaluation of quick intraoperative parathyroid hormone assay at the time of skin closure in predicting clinically relevant hypocalcemia after thyroidectomy. World J Surg 2012;36:1300–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cavicchi O, Piccin O, Caliceti U, Fernandez IJ, Bordonaro C, Saggese D et al. Accuracy of PTH assay and corrected calcium in early prediction of hypoparathyroidism after thyroid surgery. Otolaryngol Head Neck Surg 2008;138:594–600 [DOI] [PubMed] [Google Scholar]
- 111. Schlottmann F, Arbulu AL, Sadava EE, Mendez P, Pereyra L, Fernandez Vila JM et al. Algorithm for early discharge after total thyroidectomy using PTH to predict hypocalcemia: prospective study. Langenbecks Arch Surg 2015;400:831–836 [DOI] [PubMed] [Google Scholar]
- 112. Vescan A, Witterick I, Freeman J. Parathyroid hormone as a predictor of hypocalcemia after thyroidectomy. The Laryngoscope 2005;115:2105–2108 [DOI] [PubMed] [Google Scholar]
- 113. Noureldine SI, Genther DJ, Lopez M, Agrawal N, Tufano RP. Early predictors of hypocalcemia after total thyroidectomy: an analysis of 304 patients using a short-stay monitoring protocol. JAMA Otolaryngol Head Neck Surg 2014;140:1006–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sahli Z, Najafian A, Kahan S, Schneider EB, Zeiger MA, Mathur A. One-hour postoperative parathyroid hormone levels do not reliably predict hypocalcemia after thyroidectomy. World J Surg 2018;42:2128–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bashir AY, Alzubaidi AN, Bashir MA, Obed AH, Zakarneh RK, Ennab HZ et al. The optimal parathyroid hormone cut-off threshold for early and safe management of hypocalcemia after total thyroidectomy. Endocr Practice 2021;27:925–933 [DOI] [PubMed] [Google Scholar]
- 116. Cote V, Sands N, Hier MP, Black MJ, Tamilia M, MacNamara E XZ et al. Cost savings associated with post-thyroidectomy parathyroid hormone levels. Otolaryngol Head Neck Surg 2008;138:204–208 [DOI] [PubMed] [Google Scholar]
- 117. Warren FM, Andersen PE, Wax MK, Cohen JI. Intraoperative parathyroid hormone levels in thyroid and parathyroid surgery. The Laryngoscope 2002;112:1866–1870 [DOI] [PubMed] [Google Scholar]
- 118. Chia SH, Weisman RA, Tieu D, Kelly C, Dillmann WH, Orloff LA. Prospective study of perioperative factors predicting hypocalcemia after thyroid and parathyroid surgery. Arch Otolaryngol Head Neck Surg 2006;132:41–45 [DOI] [PubMed] [Google Scholar]
- 119. Erbil Y, Bozbora A, Ozbey N, Issever H, Aral F, Ozarmagan S et al. Predictive value of age and serum parathormone and vitamin d3 levels for postoperative hypocalcemia after total thyroidectomy for nontoxic multinodular goiter. Arch Surg 2007;142:1182–1187 [DOI] [PubMed] [Google Scholar]
- 120. Lombardi CP, Raffaelli M, Princi P, Dobrinja C, Carrozza C, Di Stasio E et al. Parathyroid hormone levels 4 h after surgery do not accurately predict post-thyroidectomy hypocalcemia. Surgery 2006;140:1016–1025 [DOI] [PubMed] [Google Scholar]
- 121. Warren FM, Andersen PE, Wax MK, Cohen JI. Perioperative parathyroid hormone levels in thyroid surgery: preliminary report. Laryngoscope 2004;114:689–693 [DOI] [PubMed] [Google Scholar]
- 122. Al Khadem MG, Rettig EM, Dhillon VK, Russell JO, Tufano RP. Postoperative IPTH compared with IPTH gradient as predictors of post-thyroidectomy hypocalcemia. Laryngoscope 2018;128:769–774 [DOI] [PubMed] [Google Scholar]
- 123. Albuja-Cruz MB, Pozdeyev N, Robbins S, Chandramouli R, Raeburn CD, Klopper J et al. A safe and effective protocol for management of post-thyroidectomy hypocalcemia. Am J Surg 2015;210:1162–1169 [DOI] [PubMed] [Google Scholar]
- 124. Inversini D, Rausei S, Ferrari CC, Frattini F, Anuwong A, Kim HY et al. Early intact PTH (iPTH) is an early predictor of postoperative hypocalcemia for a safer and earlier hospital discharge: an analysis on 260 total thyroidectomies. Gland Surg 2016;5:522–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Raffaelli M, De Crea C, Carrozza C, D’Amato G, Zuppi C, Bellantone R et al. Combining early postoperative parathyroid hormone and serum calcium levels allows for an efficacious selective post-thyroidectomy supplementation treatment. World J Surg 2012;36:1307–1313 [DOI] [PubMed] [Google Scholar]
- 126. Grodski S, Lundgren CI, Sidhu S, Sywak M, Delbridge L. Postoperative PTH measurement facilitates day 1 discharge after total thyroidectomy. Clin Endocrinol 2009;70:322–325 [DOI] [PubMed] [Google Scholar]
- 127. Mehrvarz S, Mohebbi HA, Motamedi MH, Khatami SM, Rezaie R, Rasouli HR. Parathyroid hormone measurement in prediction of hypocalcaemia following thyroidectomy. J Coll Physicians and Surg Pak 2014;24:82–87 [PubMed] [Google Scholar]
- 128. Galy-Bernadoy C, Lallemant B, Chambon G, Pham HT, Reynaud C, Alovisetti C et al. Parathyroid hormone assays following total thyroidectomy: is there a predictive value? Eur Thyroid J 2018;7:34–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kolahdouzan M, Shahmiri SS, Hashemi SM, Keleidari B, Nazem M, Mofrad RM. Is decline rate of intact parathyroid hormone level a reliable criterion for early discharge of patients after total thyroidectomy? Iran J Otorhinolaryngol 2017;29:239–246 [PMC free article] [PubMed] [Google Scholar]
- 130. Reddy AC, Chand G, Sabaretnam M, Mishra A, Agarwal G, Agarwal A et al. Prospective evaluation of intra-operative quick parathyroid hormone assay as an early predictor of post thyroidectomy hypocalcaemia. Int J Surg 2016;34:103–108 [DOI] [PubMed] [Google Scholar]
- 131. Payne RJ, Tewfik MA, Hier MP, Tamilia M, Namara EM, Young J et al. Benefits resulting from 1-and 6-h parathyroid hormone and calcium levels after thyroidectomy. Otolaryngol Head Neck Surg 2005;133:386–390 [DOI] [PubMed] [Google Scholar]
- 132. Al-Dhahri SF, Al-Ghonaim YA, Terkawi AS. Accuracy of postthyroidectomy parathyroid hormone and corrected calcium levels as early predictors of clinical hypocalcemia. J Otolaryngol Head Neck Surg 2010;39:342–348 [PubMed] [Google Scholar]
- 133. Lecerf P, Orry D, Perrodeau E, Lhommet C, Charretier C, Mor C et al. Parathyroid hormone decline 4 h after total thyroidectomy accurately predicts hypocalcemia. Surgery 2012;152:863–868 [DOI] [PubMed] [Google Scholar]
- 134. Higgins KM, Mandell DL, Govindaraj S, Genden EM, Mechanick JI, Bergman DA et al. The role of intraoperative rapid parathyroid hormone monitoring for predicting thyroidectomy-related hypocalcemia. Arch Otolaryngol Head Neck Surg 2004;130:63–67 [DOI] [PubMed] [Google Scholar]
- 135. Soon PS, Magarey CJ, Campbell P, Jalaludin B. Serum intact parathyroid hormone as a predictor of hypocalcaemia after total thyroidectomy. ANZ J Surg 2005;75:977–980 [DOI] [PubMed] [Google Scholar]
- 136. Asari R, Passler C, Kaczirek K, Scheuba C, Niederle B. Hypoparathyroidism after total thyroidectomy: a prospective study. Arch Surg 2008;143:132–137 [DOI] [PubMed] [Google Scholar]
- 137. Cranshaw IM, Moss D, Whineray-Kelly E, Harman CR. Intraoperative parathormone measurement from the internal jugular vein predicts post-thyroidectomy hypocalcaemia. Langenbecks Arch Surg 2007;392:699–702 [DOI] [PubMed] [Google Scholar]
- 138. Di Fabio F, Casella C, Bugari G, Iacobello C, Salerni B. Identification of patients at low risk for thyroidectomy-related hypocalcemia by intraoperative quick PTH. World J Surg 2006;30:1428–1433 [DOI] [PubMed] [Google Scholar]
- 139. Nahas ZS, Farrag TY, Lin FR, Belin RM, Tufano RP. A safe and cost-effective short hospital stay protocol to identify patients at low risk for the development of significant hypocalcemia after total thyroidectomy. Laryngoscope 2006;116:906–910 [DOI] [PubMed] [Google Scholar]
- 140. Lindblom P, Westerdahl J, Bergenfelz A. Low parathyroid hormone levels after thyroid surgery: a feasible predictor of hypocalcemia. Surgery 2002;131:515–520 [DOI] [PubMed] [Google Scholar]
- 141. Payne RJ, Hier MP, Tamilia M, Mac Namara E, Young J, Black MJ. Same-day discharge after total thyroidectomy: the value of 6-h serum parathyroid hormone and calcium levels. Head Neck 2005;27:1–7 [DOI] [PubMed] [Google Scholar]
- 142. Lazard DS, Godiris-Petit G, Wagner I, Sarfati E, Chabolle F. Early detection of hypocalcemia after total/completion thyroidectomy: routinely usable algorithm based on serum calcium level. World J Surg 2012;36:2590–2597 [DOI] [PubMed] [Google Scholar]
- 143. Kara M, Tellioglu G, Krand O, Fersahoglu T, Berber I, Erdogdu E et al. Predictors of hypocalcemia occurring after a total/near total thyroidectomy. Surg Today 2009;39:752–757 [DOI] [PubMed] [Google Scholar]
- 144. Pfleiderer AG, Ahmad N, Draper MR, Vrotsou K, Smith WK. The timing of calcium measurements in helping to predict temporary and permanent hypocalcaemia in patients having completion and total thyroidectomies. Ann R Coll Surg Engl 2009;91:140–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Gulluoglu BM, Manukyan MN, Cingi A, Yegen C, Yalin R, Aktan AO. Early prediction of normocalcemia after thyroid surgery. World J Surg 2005;29:1288–1293 [DOI] [PubMed] [Google Scholar]
- 146. Husein M, Hier MP, Al-Abdulhadi K, Black M. Predicting calcium status post thyroidectomy with early calcium levels. Otolaryngol Head Neck Surg 2002;127:289–293 [DOI] [PubMed] [Google Scholar]
- 147. Houette A, Massoubre J, Pereira B, Puechmaille M, Dissard A, Gilain L et al. Early corrected serum calcium value can predict definitive calcium serum level after total thyroidectomy in asymptomatic patients. Eur Arch Oto-rhino-laryngol 2018;275:2373–2378 [DOI] [PubMed] [Google Scholar]
- 148. Abdel-Halim CN, Rejnmark L, Nielsen VE. Post-operative parathyroid hormone can be used as a predictor of normocalcaemia after total thyroidectomy. Dan Med J 2015;62:A5157. [PubMed] [Google Scholar]
- 149. Islam S, Al Maqbali T, Howe D, Campbell J. Hypocalcaemia following total thyroidectomy: early post-operative parathyroid hormone assay as a risk stratification and management tool. J Laryngol Otol 2014;128:274–278 [DOI] [PubMed] [Google Scholar]
- 150. Le TN, Kerr PD, Sutherland DE, Lambert P. Validation of 1-h post-thyroidectomy parathyroid hormone level in predicting hypocalcemia. J Otolaryngol 2014;43:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Graciano AJ, Chone CT, Fischer CA. Applicability of immediate, late or serial intact parathyroid hormone measurement following total thyroidectomy. Braz J Otorhinolaryngol 2012;78:78–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Bentrem DJ, Rademaker A, Angelos P. Evaluation of serum calcium levels in predicting hypoparathyroidism after total/near-total thyroidectomy or parathyroidectomy. Am Surg 2001;67:249–251 [PubMed] [Google Scholar]
- 153. Osborne J, Papachristos A, Skandarajah A, Gorelik A, Hng D, Miller J. Selective prophylactic calcium supplementation reduces length of stay after total thyroidectomy. World J Endocr Surg 2017;9:88–93 [Google Scholar]
- 154. Wang J, Gu J, Han Q, Wang W, Shang J. Value of intraoperative parathyroid hormone monitoring in papillary thyroid cancer surgery: can it be used to guide the choice of operation methods? Int J Clin Exp Med 2015;8:7778–7785 [PMC free article] [PubMed] [Google Scholar]
- 155. Algarni M, Alzahrani R, Dionigi G, Hadi AH, AlSubayea H. Parathyroid hormone and serum calcium levels measurements as predictors of postoperative hypocalcemia in total thyroidectomy. Gland Surg 2017;6:428–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Wu SD, Gao L. Is routine calcium supplementation necessary in patients undergoing total thyroidectomy plus neck dissection? Surgery Today 2011;41:183–188 [DOI] [PubMed] [Google Scholar]
- 157. Moore C, Lampe H, Agrawal S. Predictability of hypocalcemia using early postoperative serum calcium levels. J Otolaryngol 2001;30:266–270 [DOI] [PubMed] [Google Scholar]
- 158. Ma LWY, Wong KP, Lang B. Determining the optimal time to obtain parathyroid hormone after thyroidectomy. Surg Practice 2018;22 [Google Scholar]
- 159. Abo Elwafa WA, Nabawi AS, Al Wagih HF, Fayad MH. Delta calcium as a reliable predictor of early post thyroidectomy hypocalcaemia for early safe discharge. Langenbecks Arch Surg 2017;402:362 [Google Scholar]
- 160. Graziani JG, Guerin CG, Paladino NCP, Slotema ES, Rochette CR, Romain FR et al. Predicting postoperative hypocalcemia after total thyroidectomy: reducing hospital stay, keeping the patient safe. Langenbecks Arch Surg 2017;402:391–392 [Google Scholar]
- 161. Pomeranz CL, Al-Qurayshi Z, Mohamed H, Aslam R, Friedlander P, Kandil E. Intraoperative PTH levels are predictive of post-operative hypocalcemia. Thyroid 2015;25:21125375817 [Google Scholar]
- 162. Bove A, Bongarzoni G, Di renzo R, Corradetti L, Delli santi I, Mattei PA et al. Optimal timing for PTH measurement as a predictor of hypocalcemia after total thyroidectomy. Thyroid 2009;19:S86 [Google Scholar]
- 163. Chang JW, Park KW, Jung SN, Liu L, Kim SM, Koo BS. The most reliable time point for intact parathyroid hormone measurement to predict hypoparathyroidism after total thyroidectomy with central neck dissection to treat papillary thyroid carcinoma: a prospective cohort study. Eur Arch Otorhinolaryngol. 2020;277:549–558 [DOI] [PubMed] [Google Scholar]
- 164. Al-Dhahri SF, Mubasher M, Al-Muhawas F, Alessa M, Terkawi RS, Terkawi AS. Early prediction of oral calcium and vitamin D requirements in post-thyroidectomy hypocalcaemia. Otolaryngol Head Neck Surg 2014;151:407–414 [DOI] [PubMed] [Google Scholar]
- 165. Lim JP, Irvine R, Bugis S, Holmes D, Wiseman SM. Intact parathyroid hormone measurement 1 h after thyroid surgery identifies individuals at high risk for the development of symptomatic hypocalcemia. Am J Surg 2009;197:648–654 [DOI] [PubMed] [Google Scholar]
- 166. Youngwirth L, Benavidez J, Sippel R, Chen H. Postoperative parathyroid hormone testing decreases symptomatic hypocalcemia and associated emergency room visits after total thyroidectomy. Surgery 2010;148:841–846 [DOI] [PubMed] [Google Scholar]
- 167. Moriyama T, Yamashita H, Noguchi S, Takamatsu Y, Ogawa T, Watanabe S et al. Intraoperative parathyroid hormone assay in patients with Graves’ disease for prediction of postoperative tetany. World J Surg 2005;29:1282–1287 [DOI] [PubMed] [Google Scholar]
- 168. Kala F, Sarici IS, Ulutas KT, Sevim Y, Dogu A, Sarigoz T et al. Intact parathormone measurement 1 h after total thyroidectomy as a predictor of symptomatic hypocalcemia. Int J Clin Exp Med 2015;8:18813–18818 [PMC free article] [PubMed] [Google Scholar]
- 169. Lee JW, Kim JK, Kwon H, Lim W, Moon BI, Paik NS. Routine low-dose calcium supplementation after thyroidectomy does not reduce the rate of symptomatic hypocalcemia: a prospective randomized trial. Ann Surg Treat Res 2019;96:177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Mo K, Shang J, Wang K, Gu J, Wang P, Nie X et al. Parathyroid hormone reduction predicts transient hypocalcemia after total thyroidectomy: a single-center prospective study. Int J Endocrinol 2020;2020:7189857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Mazotas IG, Wang TS. The role and timing of parathyroid hormone determination after total thyroidectomy. Gland Surg 2017;6:S38–s48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Jr BC S, Bimston DN, Bodenner DL, Brett EM, Dralle H, Orloff LA et al. American association of clinical endocrinologists and American college of endocrinology disease state clinical review: postoperative hypoparathyroidism-definitions and management. Endocr Practice 2015;21:674–685 [DOI] [PubMed] [Google Scholar]
- 173. Grodski S, Serpell J. Evidence for the role of perioperative PTH measurement after total thyroidectomy as a predictor of hypocalcemia. World J Surg 2008;32:1367–1373 [DOI] [PubMed] [Google Scholar]
- 174. Paladino NC, Guérin C, Graziani J, Morange I, Loundou A, Taïeb D et al. Predicting risk factors of postoperative hypocalcemia after total thyroidectomy: is safe discharge without supplementation possible? A large cohort study. Langenbecks Arch Surg 2021;406:2425–2431 [DOI] [PubMed] [Google Scholar]
- 175. Bilezikian JP, Khan A, Potts JT Jr, Brandi ML, Clarke BL, Shoback D et al. Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Mineral Res 2011;26:2317–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Croix CL, Potard G, Valette G, Marianowski R. Interest of the parathyroid hormone assay: an early predictor of post-thyroidectomy hypocalcemia. Otolaryngol Head Neck Surg 2014;151:P171 [Google Scholar]
- 177. Payne RJ, Hier MP, Tamilia M, Young J, MacNamara E, Black MJ. Postoperative parathyroid hormone level as a predictor of post-thyroidectomy hypocalcemia. J Otolaryngol 2003;32:362–367 [DOI] [PubMed] [Google Scholar]
- 178. Brandi ML, Bilezikian JP, Shoback D, Bouillon R, Clarke BL, Thakker RV et al. Management of hypoparathyroidism: summary statement and guidelines. J Clin Endocrinol Metab 2016;101:2273–2283 [DOI] [PubMed] [Google Scholar]
- 179. Pelizzo MR, Piotto A, Toniato A, Pagetta C. PTH assay in the first postoperative day after thyroidectomy early predictor postoperative hypocalcemia?. Ann Ital Chir 2003;74:511–515 [PubMed] [Google Scholar]
- 180. Mazotas IG, Yen TWF, Park J, Liu Y, Eastwood DC, Carr AA et al. A postoperative parathyroid hormone-based algorithm to reduce symptomatic hypocalcemia following completion/total thyroidectomy: a retrospective analysis of 591 patients. Surgery U S 2018;164:746–753 [DOI] [PubMed] [Google Scholar]
- 181. Quiros RM, Pesce CE, Wilhelm SM, Djuricin G, Prinz RA. Intraoperative parathyroid hormone levels in thyroid surgery are predictive of postoperative hypoparathyroidism and need for vitamin D supplementation. Am J Surg 2005;189:306–309 [DOI] [PubMed] [Google Scholar]
- 182. Riaz U, Shah SA, Zahoor I, Riaz A, Zubair M. Validity of early parathyroid hormone assay as a diagnostic tool for sub-total thyroidectomy related hypocalcaemia. J Coll Physicians Surg Pak 2014;24:459–462 [PubMed] [Google Scholar]
- 183. Kovacevic B, Ignjatovic M, Cuk V, Zivaljevic V, Paunovic I. Early prediction of symptomatic hypocalcemia after total thyroidectomy. Acta Chir Belg 2011;111:303–307 [PubMed] [Google Scholar]
- 184. Kim DS, Wang RC. 92. Otolaryngol Head Neck Surg 2016; 155: P38. [Google Scholar]
- 185. Rubin SJ, Park JH, Pearce EN, Holick MF, McAneny D, Noordzij JP. Vitamin D status as a predictor of postoperative hypocalcemia after thyroidectomy. Otolaryngol Head Neck Surg 2020;163:501–507 [DOI] [PubMed] [Google Scholar]
- 186. Kim WW, Chung SH, Ban EJ, Lee CR, Kang SW, Jeong JJ et al. Is preoperative vitamin D deficiency a risk factor for postoperative symptomatic hypocalcemia in thyroid cancer patients undergoing total thyroidectomy plus central compartment neck dissection? Thyroid 2015;25:911–918 [DOI] [PubMed] [Google Scholar]
- 187. Erbil Y, Barbaros U, Temel B, Turkoglu U, Işsever H, Bozbora A et al. The impact of age, vitamin D(3) level, and incidental parathyroidectomy on postoperative hypocalcemia after total or near total thyroidectomy. Am J Surg 2009;197:439–446 [DOI] [PubMed] [Google Scholar]
- 188. Al-Khatib T, Althubaiti AM, Althubaiti A, Mosli HH, Alwasiah RO, Badawood LM. Severe vitamin D deficiency: a significant predictor of early hypocalcemia after total thyroidectomy. Otolaryngol Head Neck Surg 2015;152:424–431 [DOI] [PubMed] [Google Scholar]
- 189. Díez M, Vera C, Ratia T, Diego L, Mendoza F, Guillamot P et al. [Effect of vitamin D deficiency on hypocalcaemia after total thyroidectomy due to benign goitre]. Cirugia Espanola 2013;91:250–256 [DOI] [PubMed] [Google Scholar]
- 190. Kirkby-Bott J, Markogiannakis H, Skandarajah A, Cowan M, Fleming B, Palazzo F. Preoperative vitamin D deficiency predicts postoperative hypocalcemia after total thyroidectomy. World J Surg 2011;35:324–330 [DOI] [PubMed] [Google Scholar]
- 191. Wang X, Zhu J, Liu F, Gong Y, Li Z. Preoperative vitamin D deficiency and postoperative hypocalcemia in thyroid cancer patients undergoing total thyroidectomy plus central compartment neck dissection. Oncotarget 2017;8:78113–78119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Godazandeh G, Kashi Z, Godazandeh F, Tayebi P, Bijani A. Influence of thyroidectomy on postoperative serum calcium level regarding serum vitamin D status. A prospective study. Caspian J Intern Med 2015;6:72–76 [PMC free article] [PubMed] [Google Scholar]
- 193. Lee GH, Ku YH, Kim HI, Lee MC, Kim MJ. Vitamin D level is not a predictor of hypocalcemia after total thyroidectomy. Langenbecks Arch Surg 2015;400:617–622 [DOI] [PubMed] [Google Scholar]
- 194. Erlem M, Klopp-Dutote N, Biet-Hornstein A, Strunski V, Page C. Impact of pre-operative serum 25-hydroxyvitamin D on post-operative serum calcium in patients undergoing total thyroidectomy for benign goitre: retrospective study of 246 patients. J Laryngol Otol 2017;131:925–929 [DOI] [PubMed] [Google Scholar]
- 195. Lang BH, Wong KP, Cowling BJ, Fong YK, Chan DK, Hung GK. Do low preoperative vitamin D levels reduce the accuracy of quick parathyroid hormone in predicting postthyroidectomy hypocalcemia? Ann Surg Oncol 2013;20:739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Lin Y, Ross HL, Raeburn CD, DeWitt PE, Albuja-Cruz M, Jones EL et al. Vitamin D deficiency does not increase the rate of postoperative hypocalcemia after thyroidectomy. Am J Surg 2012;204:888–894 [DOI] [PubMed] [Google Scholar]
- 197. Falcone TE, Stein DJ, Jumaily JS, Pearce EN, Holick MF, McAneny DB et al. Correlating pre-operative vitamin D status with post-thyroidectomy hypocalcemia. Endocr Practice 2015;21:348–354 [DOI] [PubMed] [Google Scholar]
- 198. Griffin TP, Murphy MS, Sheahan P. Vitamin D and risk of postoperative hypocalcemia after total thyroidectomy. JAMA Otolaryngol Head Neck Surg 2014;140:346–351 [DOI] [PubMed] [Google Scholar]
- 199. Cherian AJ, Ponraj S, Gowri SM, Ramakant P, Paul TV, Abraham DT et al. The role of vitamin D in post-thyroidectomy hypocalcemia: Still an enigma. Surgery 2016;159:532–538 [DOI] [PubMed] [Google Scholar]
- 200. Musholt TJ, Clerici T, Dralle H, Frilling A, Goretzki PE, Hermann MM; German Association of Endocrine Surgeons practice guidelines for the surgical treatment of benign thyroid disease ; Arch Surg 2011;396:639–49 [DOI] [PubMed] [Google Scholar]
- 201. Sharif SB, Rakib SA, Naznin KS, Alam SM. Early postoperative parathyroid hormone level as a predictor of hypocalcaemia after total thyroidectomy. Mymensingh Med J MMJ 2017;26:335–340 [PubMed] [Google Scholar]
- 202. Rivere AE, Brooks AJ, Hayek GA, Wang H, Corsetti RL, Fuhrman GM. Parathyroid hormone levels predict posttotal thyroidectomy hypoparathyroidism. Am Surg 2014;80:817–820 [PubMed] [Google Scholar]
- 203. Zhang S. The application value of parathyroid hormone level in predicting post-operative hypocalcemia after total thyroidectomy. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2016;30:39–41 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of this review and meta-analysis can be made available to any researcher. All relevant data are included in the tables and supplemental material. All other material and data can be provided directly on request to the corresponding author.