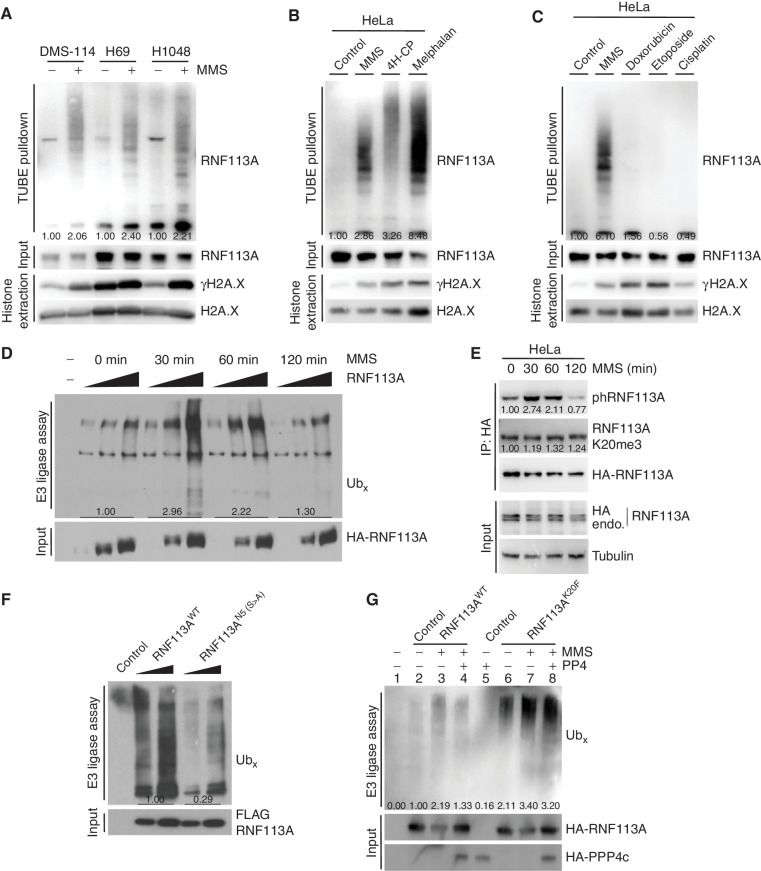
Figure 5.
Methylation–phosphorylation cross-talk regulation of RNF113A affects its E3 ligase activity. A, Immunodetection of autoubiquitinated RNF113A after TUBE pulldowns using DMS-114, H69, and H1048 SCLC cell extracts following treatment with MMS. γH2A.X is shown as a marker of DNA damage induction. B, Immunodetection of autoubiquitinated RNF113A after TUBE pulldowns as in A, using HeLa cells extracts after treatment with different alkylating agents. γH2A.X is shown as a marker of DNA damage induction. C, Immunodetection of autoubiquitinated RNF113A after TUBE pulldowns as in A, using HeLa cells extracts after treatment with MMS versus different nonalkylating DNA-damaging agents. γH2A.X is shown as a marker of DNA damage induction. D,In vitro E3 ubiquitin ligase assays were performed with FLAG-HA-RNF113A purified from HeLa S3 cells with or without prior MMS treatment for the indicated duration. This was followed by immunoblot analysis with the indicated antibodies. E, Immunoblot analysis of total, phosphorylated, and methylated RNF113A immunoprecipitated from HeLa cells stably expressing HA-RNF113A, with or without prior MMS treatment for the indicated duration. F,In vitro E3 ubiquitin ligase assays were performed with WT or N5-mutant forms of RNF113A purified from HeLa S3 cells. This was followed by immunoblot analysis with the indicated antibodies. G,In vitro E3 ubiquitin ligase assays were performed as in F using WT or K20F-mutant forms of RNF113A purified from HeLa S3 cells treated with or without prior MMS treatment. Where indicated the E3 enzyme was preincubated with PP4 phosphatase. In all panels, representative of at least three independent experiments is shown unless stated otherwise. The numbers below the immunoblot lines represent the relative signal quantification (see also Supplementary Table S5).