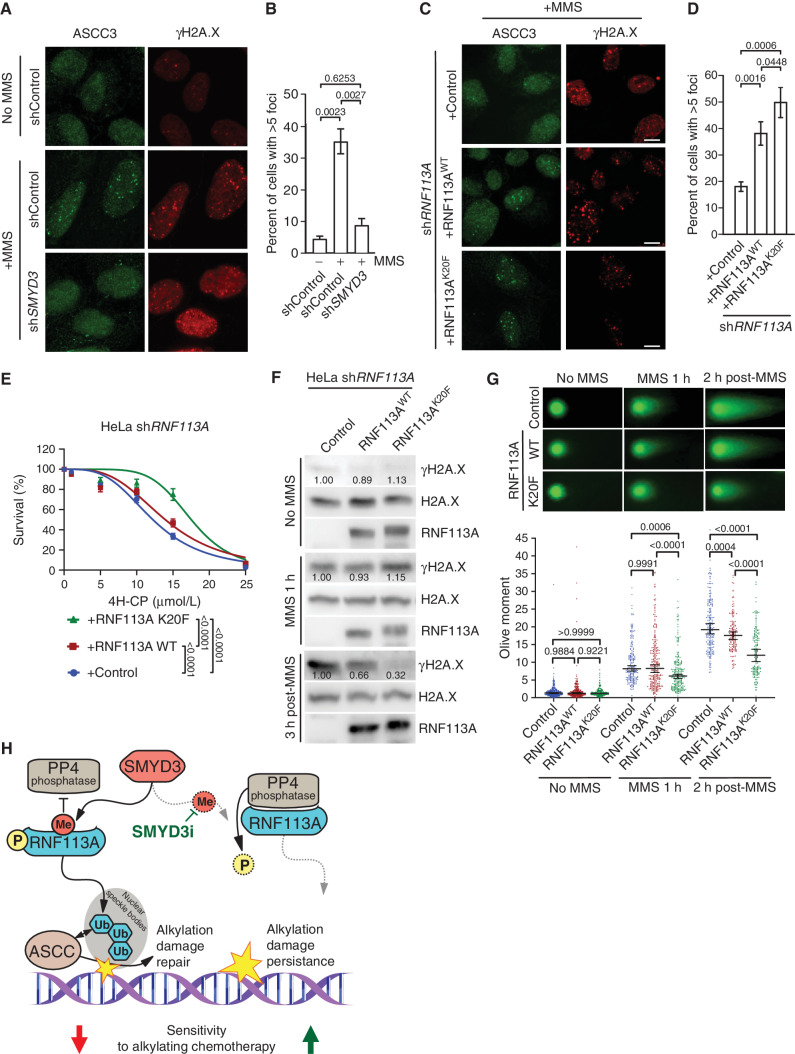
Figure 6.
RNF113A regulation affects its function in DNA dealkylation repair. A, Representative images of MMS-induced ASCC3 foci in shSMYD3 or shControl U2OS cells with or without prior MMS. Foci were monitored by immunofluorescent staining of ASCC3 (left) and the DNA damage marker γH2A.X (right). B, Quantification of ASCC3 foci formation from A. A minimum of 100 cells were quantified for each experimental condition. P values were calculated by a two-tailed unpaired Student t test, and error bars represent mean ± SD. C, Representative images of MMS-induced ASCC3 foci as in A in U2OS cells reconstituted with either RNF113A WT or K20F mutant after endogenous RNF113A knockdown by shRNA (shRNF113A). D, Quantification of ASCC3 foci formation from C. A minimum of 100 cells were counted for each experimental condition. P values were calculated by two-tailed unpaired Student t test, and error bars represent mean ± SD. E, Engineered HeLa cell viability assays using different concentrations of 4H-CP. Cells were stably transduced with inducible shRNA RNF113A (shRNF113A) and reconstituted with either WT RNF113A or the K20F mutant. The percentage of viable cells under each condition was normalized to untreated cells. Each condition represents the mean of three technical replicates from two independent experiments. P values were calculated by two-way ANOVA with Tukey testing for multiple comparisons. Data are represented as nonlinear regression with mean ± SEM. F, Immunoblots with indicated antibodies of cell lysates as in E with or without MMS treatment for the indicated duration and with or without the indicated recovery duration. G, Neutral comet assays depicting DNA double-stranded break repair in engineered HeLa cells as in F with representative examples of comet tails (top) and Olive moment quantification (bottom). A minimum of 150 comets were analyzed for each condition. P values were calculated by two-way ANOVA with the Tukey test for multiple comparisons. Data are represented as median with 95% CI. H, Model of SMYD3 participation in coordinating SCLC response to alkylating therapy through RNF113A methylation. In SCLC overexpressing SMYD3 (left), RNF113A activation leads to efficient dealkylation repair by ASCC and loss of cancer sensitivity to alkylation-based chemotherapy. Specific SMYD3 inhibition allows for RNF113A inactivation by PP4 and prevents RNF113A-mediated alkylation damage response, leading to sustained tumor growth inhibition by alkylating chemotherapy (right). In all panels, representative of at least three independent experiments is shown unless stated otherwise. The numbers below the immunoblot lines represent the relative signal quantification (see also Supplementary Table S5).