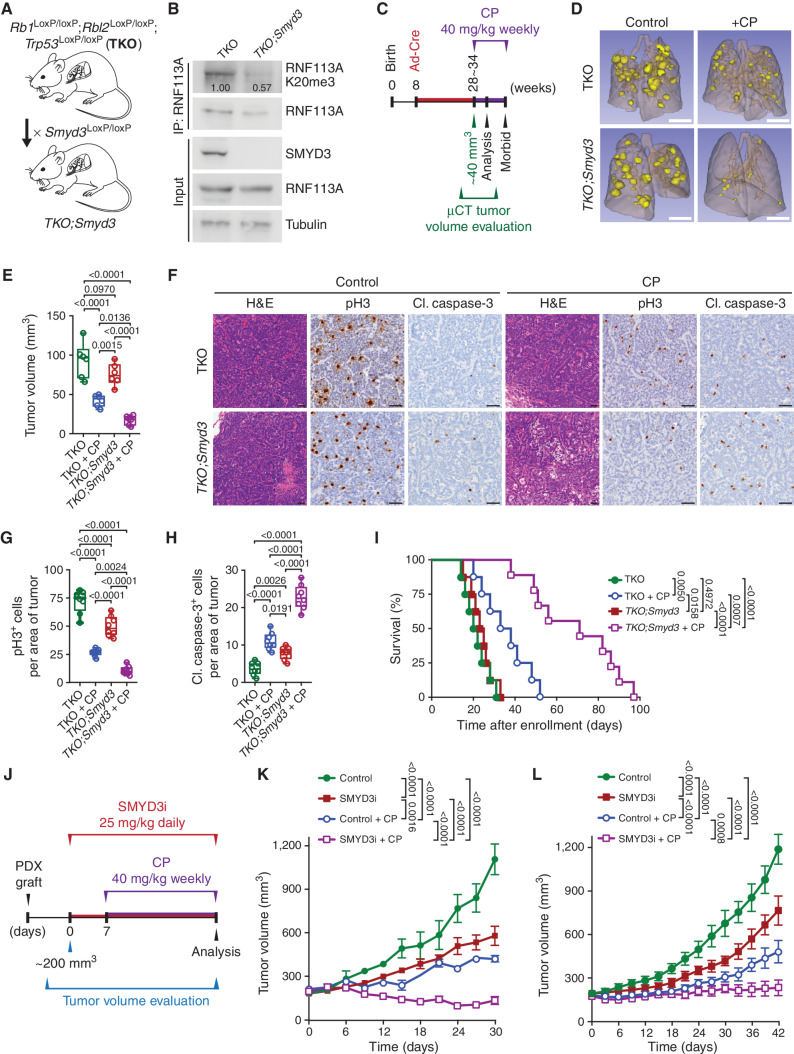
Figure 7.
SMYD3 inhibition sensitizes SCLC to alkylating agents in vivo. A, Schematic of an SCLC mouse model with conditional deletion of Rb1, Rbl2, and Trp53 (TKO) and generation of conditional Smyd3 mutant in the TKO background (TKO;Smyd3). B, Immunoblot analysis of endogenous RNF113A K20me3 methylation following immunoprecipitation of total RNF113A in cell lines originating from TKO and TKO;Smyd3-mutant mice. SMYD3 is provided as a validation of successful Smyd3 deletion in TKO;Smyd3 mice. Tubulin was used as a loading control. C, Schematic of treatment procedures to induce SCLC in TKO and TKO;Smyd3-mutant mice followed by the evaluation of therapeutic response to CP. Tumor volume was evaluated by μCT. Animals were enrolled in the study once tumor volume reached approximately 40 mm3 for TKO control animals on average at 28 and TKO;Smyd3 at 35 weeks after tumor induction. Mice cohorts were analyzed at 15 days after enrollment after receiving two rounds of CP or were continuously treated with CP or vehicle (control) until signs of morbidity to establish overall survival. D, Representative μCT scans at 15 days after enrollment in TKO and TKO;Smyd3-mutant mice treated with vehicle (control) or CP (representative of n = 6 mice for each experimental group). Scale bars, 1 cm. E, Quantification of tumor volume in TKO and TKO;Smyd3-mutant mice treated with vehicle (control) or CP. Boxes represent 25th to 75th percentiles; whiskers: min. to max.; center line: median. P values were calculated by two-way ANOVA with the Tukey test for multiple comparisons. F, Representative hematoxylin and eosin (H&E) and IHC staining for cell proliferation marker phospho-Histone 3 (pH3) and apoptosis maker cleaved caspase-3 (cl. caspase-3) of lung tissue from vehicle (control) and CP-treated TKO and TKO;Smyd3-mutant mice (representative of n = 6 mice for each experimental group). Scale bars, 50 μm. G and H, Quantification of proliferation (pH3-positive cells; G) and apoptosis (cl. caspase-3–positive cells; H) in samples as in F. Boxes represent 25th to 75th percentiles; whiskers: min. to max.; center line: median. P values were calculated by two-way ANOVA with the Tukey test for multiple comparisons. I, Kaplan–Meier survival curves of control TKO (med. survival post enrollment: 21 days, n = 8), control TKO;Smyd3 (med. survival post enrollment: 24 days, n = 8), TKO + CP treatment (med. survival post enrollment: 35.5 days, n = 8) and TKO;Smyd3 + CP treatment (med. survival post enrollment: 71 days, n = 9). P values were calculated by the log-rank test. J, Schedule protocol for SCLC PDX treatment with CP and SMYD3 inhibitor EPZ031686 (SMYD3i). Mice undergoing monotherapy also received vehicle treatment. K and L, Tumor volume quantification for patient-derived SCLC xenografts obtained from therapy-naïve (K) and treated with standard chemotherapy (carboplatin and etoposide) patient (L) grafted subcutaneously to immunocompromised NSG mice (n = 6 mice, for each treatment group). P values were calculated by two-way ANOVA with the Tukey test for multiple comparisons. Data are represented as mean ± SEM. In all panels, representative of at least three independent experiments is shown unless stated otherwise. The numbers below the immunoblot lines represent the relative signal quantification (see also Supplementary Table S5).