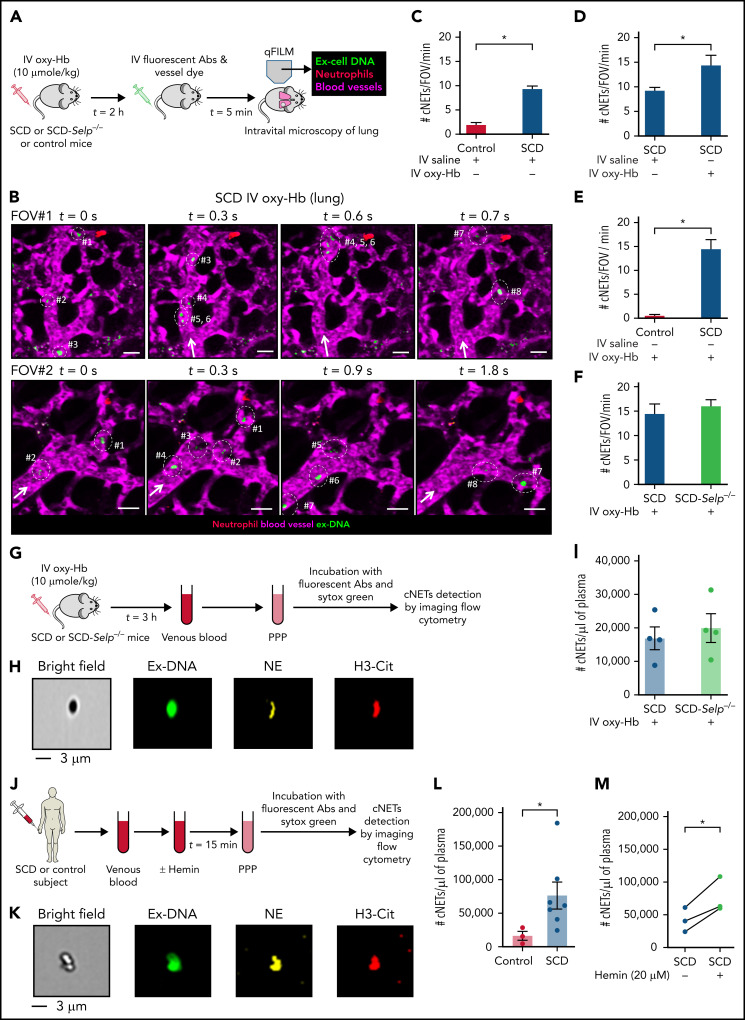
Figure 2.
Embolic NETs arrive in the lung from other organs, in a P-selectin–independent manner in SCD. (A) Experimental scheme used in panels B to E: Control, SCD, or SCD-Selp−/− mice were IV administered 10 µmol/kg oxy-Hb or saline, and qFILM was used to assess the absence or presence of cNETs within the pulmonary microcirculation. Pulmonary microcirculation (pseudo-colored purple), neutrophils (pseudo-colored red), and extracellular DNA (pseudo-colored green) were labeled in vivo by IV administration of FITC dextran, Pacific blue–anti-Ly6G Ab and Sytox orange, respectively. (B) Two representative qFILM FOVs (#1 and #2) showing several cNETs (green fragments marked with white circles) entering the lung microcirculation (purple) via the pulmonary arteriole at different time points in mice with SCD administered IV oxy-Hb. FOV#1 (top row): cNETs #1, #2, and #3 entered via the pulmonary arteriole (diameter ∼28 μm) at 0 seconds, cNETs #1, #2 left the FOV and #4, #5, #6 entered the FOV at 0.3 seconds, cNET #3 left the FOV at 0.6 seconds, cNETs #4, #5, #6 left the FOV, and #7, #8 entered the FOV at 0.7 seconds. FOV#2 (bottom row): cNETs #1, #2 entered the FOV via the pulmonary arteriole (diameter ∼24 μm) at 0 seconds, cNETs #3, #4 entered the FOV at 0.3 seconds, cNETs #1, #2, #3, #4 left the FOV and #5, #6, #7 entered the FOV at 0.9 seconds, cNETs #5, #6 left the FOV and #8 entered the FOV at 1.8 seconds. Time points are relative to the first frame shown at t = 0 seconds. Complete time series shown in supplemental Videos 8 and 9. White arrows denote the direction of blood flow within the pulmonary arterioles. Scale bars, 20 µm. (C-F) Number of cNETs entering per FOV over a 1-minute duration (#cNETs/FOV/min) were quantified using strategy described in supplemental Methods. (C) #cNETs/FOV/min were significantly more numerous in mice with SCD than control mice administered IV saline. #cNETs/FOV/min were significantly more numerous in mice with SCD administered IV oxy-Hb than (D) mice with SCD administered IV saline or (E) control mice administered IV oxy-Hb, but not different from (F) SCD-Selp−/− mice administered IV oxy-Hb. Control IV saline (n = 3 mice; 28 FOVs), SCD IV saline (n = 4 mice; 51 FOVs), SCD IV oxy-Hb (n = 4 mice; 44 FOVs), control IV Oxy-Hb (n = 4 mice, 43 FOVs), SCD-Selp−/− IV oxy-Hb (n = 5 mice; 71 FOVs). qFILM FOV size∼65 536 µm2. (G) Experimental scheme used in panels H to I: SCD or SCD-Selp−/− mice were IV administered 10 µmol/kg oxy-Hb; venous blood was processed to generate platelet poor plasma (PPP). PPP was incubated with Sytox green and fluorescent Abs against NE and citrullinated-histones (H3-Cit), and used for detection of cNETs by imaging flow cytometry as described in supplemental Methods. (H) A representative imaging flow cytometry image of a cNET in the blood of mice with SCD administered IV oxy-Hb. cNETs were identified as particles (<3 μm) triple-positive for NETs markers–extracellular DNA (green), NE (pseudo-colored yellow), and H3-Cit (pseudo-colored red). Scale bar, 3 µm. (I) Imaging flow cytometry data were quantified as described in supplemental Methods to estimate concentration of cNETs (#cNETs/μL of plasma). Plasma concentration of cNETs was not different between SCD and SCD-Selp−/− mice (n = 4 mice per group) administered IV oxy-Hb. (J) Experimental scheme used in panels K to M: control or SCD human blood with or without incubation with 20 μM hemin processed to generate PPP and cNETs detected using Imaging Flow Cytometry as in panel G. (K) A representative imaging flow cytometry image of a cNET in a patient’s blood with SCD. Scale bar, 3 µm. (L) The concentration of cNETs was significantly higher in untreated SCD (n = 7) than control (n = 3) human subjects’ blood. (M) Incubation with hemin significantly increased cNETs concentration in patients’ blood with SCD (n = 3). Straight line connects cNETs concentrations in the same patient’s blood with SCD pre- (blue circle) and post- (green circle) hemin treatment. Data in panels C-E, F, I, L represent mean ± SE and compared using Student t test. Data in panel M were compared using a paired Student t test. *P < .05.