Fig. 2 |. Hypothetical model for the neuropeptide coding of itch and pain.
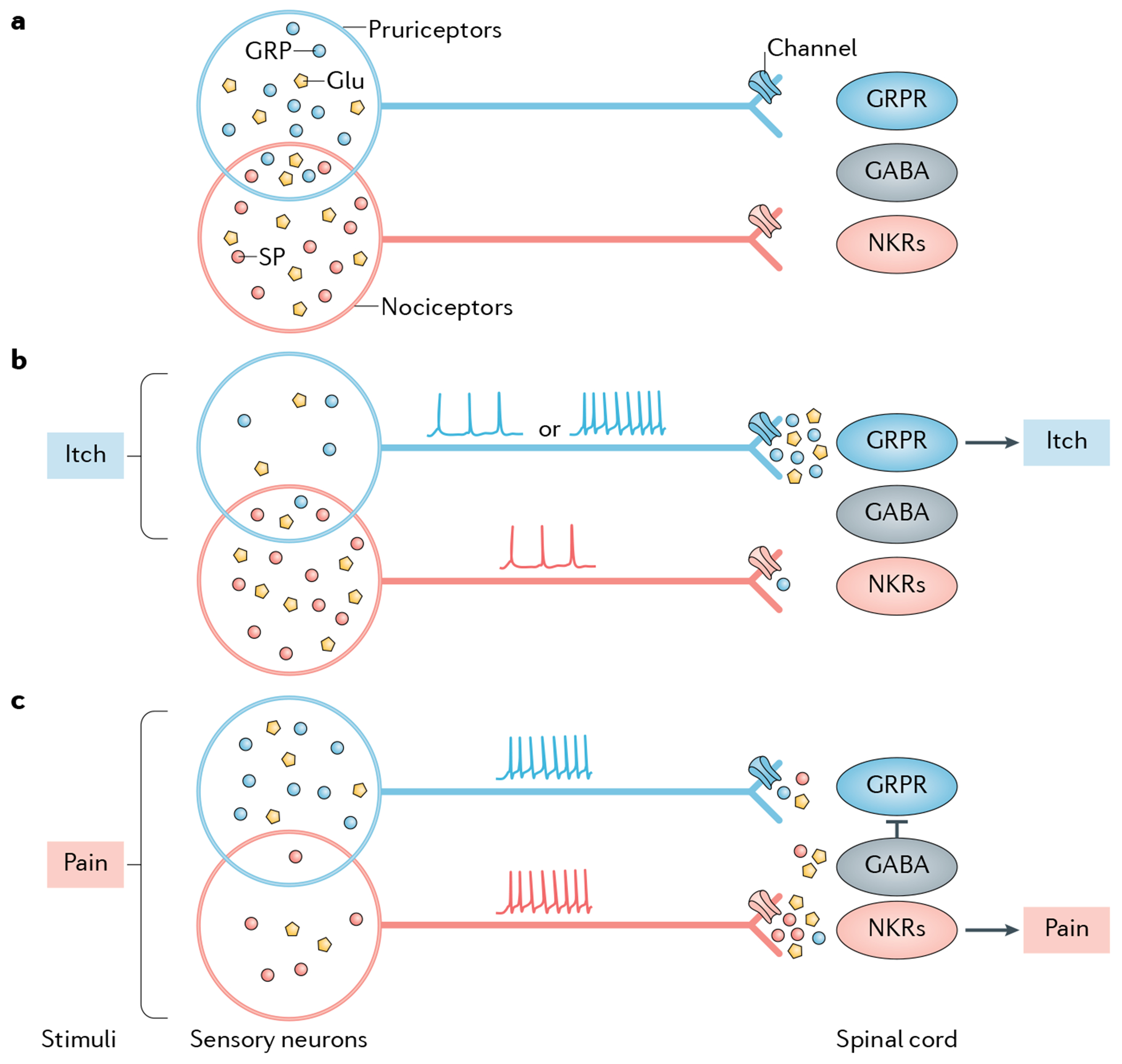
The schematics illustrate the neuropeptide code model, which suggests that itch and pain are encoded and transmitted separately through discrete neuropeptides. Substance P (SP) is shown as an example of a pain-specific neuropeptide transmitter, whereas gastrin-releasing peptide (GRP) is shown as an example of an itch-specific neuropeptide transmitter. Other types of itch-specific or pain-specific neuropeptides may exist but are not shown for illustrative purposes (see also FiG. 3). a | Pruriceptors and nociceptors partially overlap, which is manifested in co-expression of GRP and SP. In the absence of itch or pain stimuli, neither GRP nor SP is released from its terminal, resulting in a lack of itch or pain transmission. For simplistic purposes, neurotransmitters are shown only in the cell bodies of nociceptors and pruriceptors. b | Under itchy conditions, pruriceptors are activated either spontaneously (resulting in tonic firing) or in response to itch stimuli (resulting in bursting firing). This activation is proposed to release GRP and glutamate into the spinal cord, activating GRP receptor (GRPR)-expressing neurons (GRPR neurons), which transmit itch information exclusively to the brain. A smaller number of nociceptors that co-express GRP and SP (indicated as the overlap of the ovals representing these two cell populations) are also activated but fail to release SP, owing to tight control of presynaptic neurotransmitter release via channels such as sodium or calcium channels. Therefore, no pain information is transmitted. This subset of neurons may also release a small amount of GRP at synapses with spinal nociceptive neurons expressing NK receptors (NKRs), but will fail to activate them owing to the lack of GRPR in these neurons. The GABAergic inhibitory interneurons that innervate itch-specific spinal neurons remain inactive. c | Under painful conditions, a population of nociceptors that contain some pruriceptors are concomitantly activated via a bursting firing pattern that provokes the release of SP and glutamate from these neurons132,249,250, which activate spinal nociceptive neurons expressing NKRs. A small amount of GRP may be co-released but fails to activate these neurons owing to the lack of GRPR expression. Meanwhile, a smaller subset of pruriceptors expressing both GRP and SP or GRP alone (not shown) (indicated as the overlap of the ovals representing these two cell populations) is also activated and releases GRP and glutamate, which marginally activate GRPR neurons. A small amount of SP may also be co-released from pruriceptors/nociceptors via the central terminals that innervate GRPR neurons, but may fail to activate them owing to the lack of NKRs in these neurons. A concurrent activation of spinal GABAergic inhibitory neurons by SP and/or glutamate, however, inhibits the activity of GRPR neurons and thereby blocks itch transmission (see also Supplementary Fig. 1). Depending on the properties and location of the stimuli encountered, therefore, a balance between the activation of pruriceptors, nociceptors and spinal inhibitory neurons dictates whether the output of itch is completely or partially blocked. In the latter scenario, both itch information and pain information can be transmitted to the brain (not shown).
