Figure 4. Specific features, and not ubiquitination, determine cleavage.
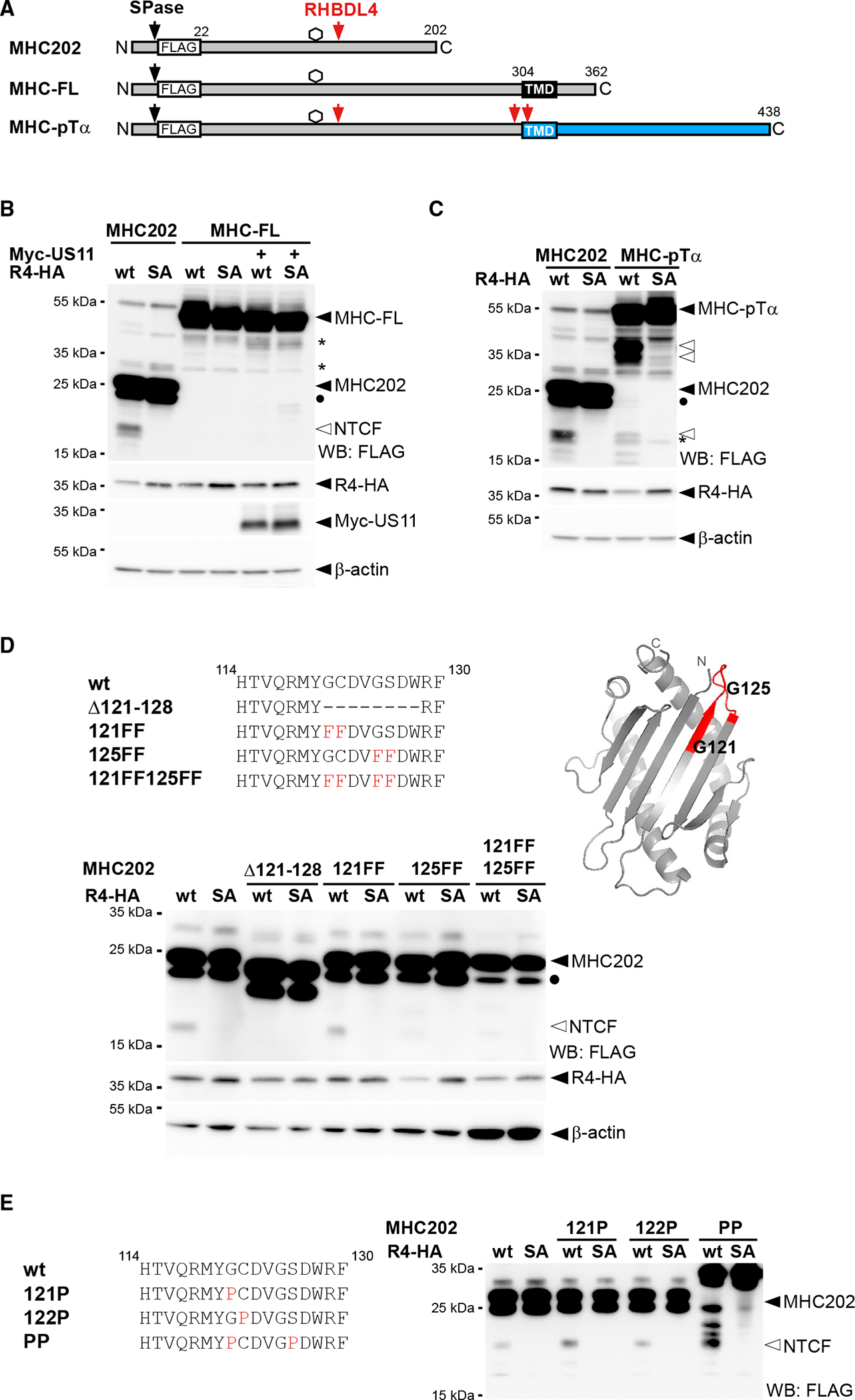
(A) Outline of MHC202, MHC-FL, and a chimera of MHC and pTα (indicated in blue). SPase, signal peptidase; TMD, transmembrane domain.
(B) The N-terminal cleavage fragment (NTCF) of MHC202 is observed in the presence of R4-HA and MG132 (2 μM) treatment, but not for MHC-FL, even upon co-expression of Myc-tagged US11. Degly., deglycosylated form of MHC202; asterisk, RHBDL4-independent degradation intermediate; WB, western blotting.
(C) Fusion to the pTα TMD degron renders MHC susceptible to RHBDL4 cleavage in the cell-based assay, as in (B).
(D) RHBDL4 cleavage assays for the indicated MHC202 deletion and point mutants were performed in the presence of MG132 (2 μM). Right: position of the two critical glycine residues (G121 and G125) and the 121–128 cleavage site region (red) in the MHC202 structure model, as shown in Figure 1A.
(E) Cleavage assay as in (D) but with MHC202 proline point mutants.
For (B)–(E) representative experiments of three biological replicates are shown. For (B) and (D), β-actin was used as a loading control.
