Figure 6. RHBDL4 targets aggregation-prone ERAD-L substrates.
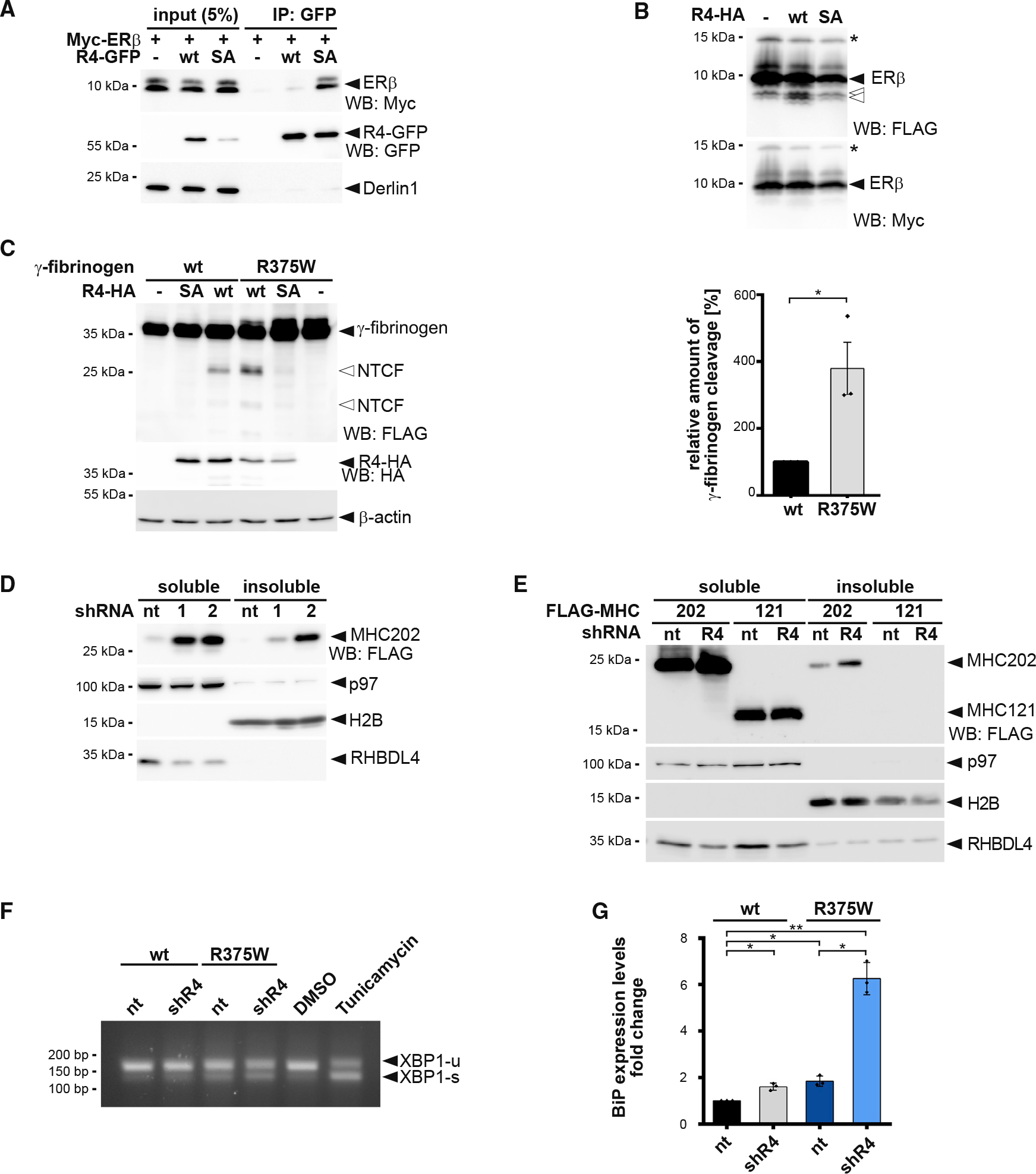
(A) N-terminal Myc-tagged ERβ interacts with the catalytic SA mutant of GFP-tagged RHBDL4 (R4-GFP) as shown by immunoprecipitation (IP), whereas no interaction is observed for the WT construct. WB, western blotting.
(B) Myc-ERβ-FLAG was co-expressed with HA-tagged RHBDL4 (R4-HA) as indicated. Tris-bicine-urea SDS-PAGE and WB analysis reveal at least two C-terminal cleavage fragments (open arrows) along with full-length ERβ. Star, undetermined post-translational modification.
(C) Mutation in fibrinogen α chain (R375W) that increases the aggregation propensity also increases generation of two N-terminal fragments (NTCF) by ectopically expressed HA-tagged RHBDL4 (R4-HA) in Hek293T cells. β-actin was used as a loading control. WB quantification is shown on the right (means ± SEM, n = 3, *p ≤ 0.05, Student’s t test).
(D) MHC202 steady-state levels in soluble and insoluble fractions are increased upon RHBDL4 knockdown by two independent shRNAs (R4–1 and R4–2) compared with non-targeting control (nt). p97 was used as a loading control for the soluble fraction and H2B for the insoluble fraction.
(E) MHC121 mimicking the RHBDL4-generated N-terminal cleavage fragment was not recovered in the NP-40 insoluble fraction, in contrast to MHC202, upon RHBDL4 shRNA knockdown (R4) compared with nt control in Hrd1 knockout cells.
(F) Simultaneous shRNA knockdown of RHBDL4 (shR4) and expression of γ-fibrinogen R375W mutant increased the UPR compared with cells co-transfected with nt control and γ-fibrinogen WT. The distribution of unspliced XBP1 (XBP1-u) and spliced XBP1 (XBP1-s) mRNA was assessed by RT-PCR. Hek293T cells were treated with tunicamycin (2 μg/mL) or DMSO for 2 h as positive or negative control, respectively.
(G) Transcriptional levels of UPR target BiP increase upon expression of γ-fibrinogen WT and R375W mutant during knockdown of RHBDL4 (shR4) compared with cells co-transfected with nt control and γ-fibrinogen WT (means ± SEM, n = 3, *p ≤ 0.05, **p ≤ 0.01, Student’s t test).
For (A)–(F), representative experiments of three biological replicates are shown.
