Fig. 1.
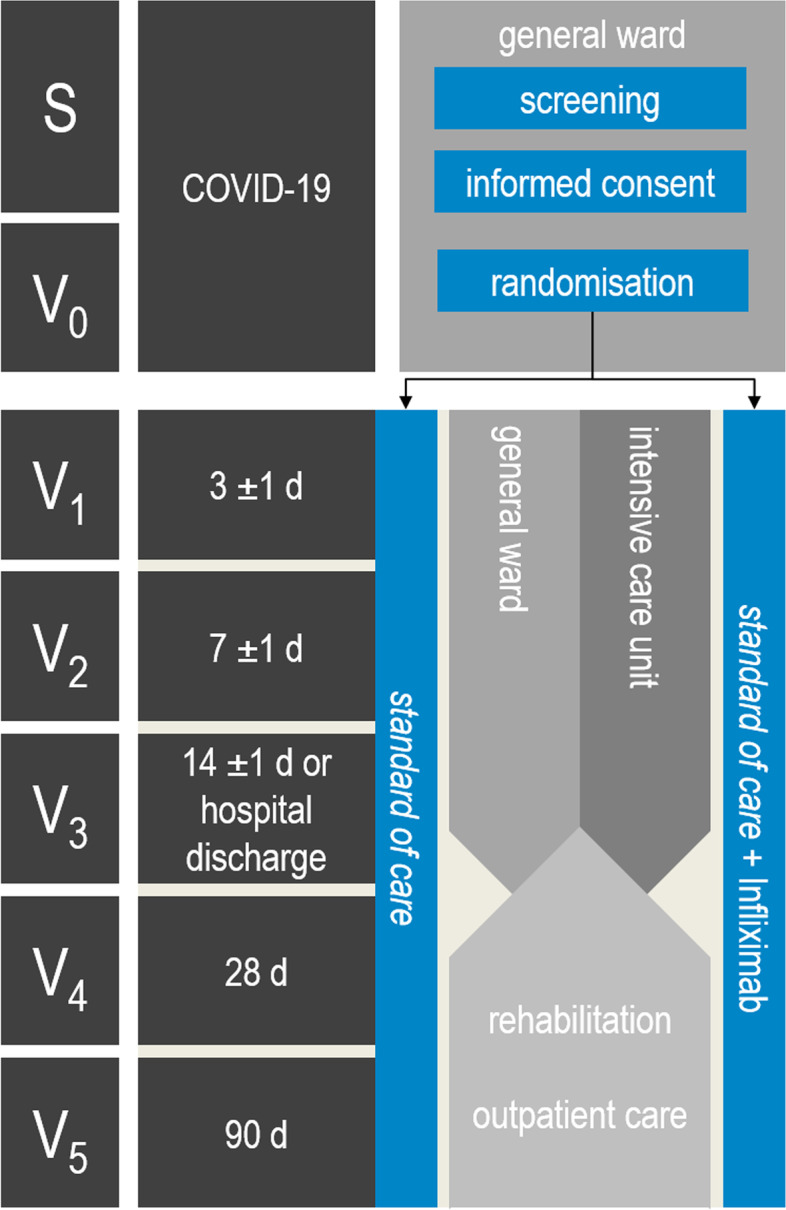
Study design. Hospitalised patients with COVID-19 (positive SARS-CoV-2 PCR testing) with pulmonary symptoms and hyperinflammation are eligible for study inclusion. Patient screening and obtainment of informed consent takes place in the general ward (S). Subsequently, patients are randomly allocated to the control (standard of care, SOC) or interventional group (SOC + infliximab) (V0). Study events take place 3 ± 1 (V1), 7 ± 1 (V2) and 14 ± 1 days after randomisation or at hospital discharge (V3). Follow-ups take place on days 28 (V4) and 90 (V5) after randomisation
