Abstract
Background and Aims:
The coronavirus disease-2019 (COVID-19) pandemic has grappled the entire globe since the beginning of 2020. In India, two vaccines were released in January 2021, the Covaxin® and the Covishield™. However, despite vaccination, many breakthrough infections were reported during the second wave in India. The present cross-sectional study aimed to find out prevalence, severity, and associated risk factors of breakthrough infection among healthcare workers (HCWs) vaccinated against COVID-19.
Material and Methods:
After ethical approval and CTRI registration, a validated questionnaire was circulated as Google form-based survey to HCWs across the nation through e-mail over 3 weeks. Biweekly reminders were sent to nonresponders till the desired sample size was attained, after which the survey was closed, and responses were charted. Data obtained from the responses were collated and analyzed.
Results:
A total of 1096 HCWs responded to the survey (54.8% response rate) and 23.36% had breakthrough infection. The severity of infection was more in the 30–50 years age group (P = 0.0170) and doctors belonging to clinical branches (P = 0.0005). The point estimate for effectiveness in preventing infection was significantly better with Covishield™ (78.5% vs. 72.4%) (P = 0.0260). Nearly all those who were infected after vaccination thought that vaccination decreased disease severity.
Conclusion:
Breakthrough COVID-19 infection still occurred after vaccination though the prevalence of severe infection was low. Covishield™ performed significantly better than Covaxin® in terms of preventing the disease. Clinical branches of medicine were found at a higher risk and younger HCWs or those with comorbidities had a higher severity of the disease.
Keywords: Coronavirus, COVID-19, health personnel, pandemics, surveys and questionnaires, vaccination, vaccines
Introduction
In the last 100 years, there have been four instances of global pandemics caused by the influenza virus; the current pandemic caused by the SARS-Cov-2 virus of the coronavirus family is one of the deadliest. Only the 1918 flu pandemic (Spanish flu) pandemic, with a total death toll of approximately 50 million (500 lakh deaths), was deadlier than this COVID-19 pandemic.[1] With a tally of 53 lakh deaths worldwide, and 4.74 lakh deaths in India (January 2020 till date) alone, and no signs of slowing down, the newly released vaccines are being thoroughly scrutinized and studied across the world by doctors and scientists to ascertain the efficacy of these vaccines by different manufacturers.[2]
In India, two vaccines were released in January 2021, the Covaxin® (Bharat Biotech; Telangana state, India) and the Covishield™ (Serum Institute of India Pvt. Ltd.; Pune, Maharashtra). The Covaxin® is an inactivated dead virus developed using the Whole-Virion Inactivated Vero Cell technology and has demonstrated an overall efficacy of 78% against COVID-19 disease.[2] Its efficacy against severe COVID-19 disease was 100% with an impact on reduction in hospitalizations.[3] Covishield™, on the other hand, is a monovalent vaccine composed of a single recombinant, replication-deficient chimpanzee adenovirus (ChAdOx1) vector encoding the S-glycoprotein of SARS-CoV-2 (ChAdOx1 nCoV-19 Corona Virus Vaccine (Recombinant); SII) and has an efficacy of 79% against symptomatic COVID-19 when the interval between doses was 4 weeks. More importantly, the efficacy in the cases of severe or critical symptomatic COVID-19 was 100%.[4]
Fifty percent of our eligible population had been fully vaccinated as of December 10, 2021, whereas 80% has received at least one dose (1,31,18,87,257 doses).[5] However, emergent circumstances in which the vaccines received approvals for mass use have brought in tentativeness in their acceptance by the general public. Since vaccination is the only definite way of diminishing the effects of the SARS-Cov-2 rampage, the pace and coverage of the inoculation must grow exponentially to achieve epidemiological significance. Prospective data on the safety and efficacy of the vaccine would help to build up confidence and increase the adoption of the vaccine by people at large, thereby would potentially help us combat the disease by achieving herd immunity. The second wave of COVID-19 witnessed a common occurrence of infection in those who had received single or double doses of either Covishield™ or Covaxin® vaccines. This reduced the trust and led to an increasing apprehension about the effectiveness of the vaccines in preventing the deadly disease. Reinfection was also a common reason to worry during the second wave. Healthcare workers (HCWs) stand at a particularly high risk of infection and were the first ones to be vaccinated after the vaccines were made available in India. It is still not clear that which of the two vaccines performs better against the virus.
We hypothesized that there is no difference in the prevalence and severity of breakthrough infection among HCWs after vaccination with both the available COVID-19 vaccines. The primary objective of this cross-sectional nationwide survey was to assess the prevalence of COVID-19 infection among the vaccinated HCWs. The secondary objectives were to find out the severity of COVID-19 infection postvaccination, the relative clinical effectiveness of Covaxin® and Covishield™ vaccines, demographic factors impacting their effectiveness, and to evaluate any risk factors for acquiring the disease postvaccination.
Material and Methods
After ethical clearance (IEC/VMMC/SJH/Project/2021-05/CC-158), and Clinical Trial Registry of India (CTRI) registration, this prospective, observational, nationwide cross-sectional survey was done. An online semistructured questionnaire was developed to assess the prevalence and severity of COVID-19 infection/reinfection among the HCWs vaccinated with either Covaxin® or Covishield™ vaccines. Breakthrough infection was defined as either a positive reverse transcription-polymerase chain reaction report, CT findings suggestive of COVID-19 infection or symptoms after exposure to a lab-confirmed case of COVID-19 infection after receiving either one or two doses of Covaxin® or Covishield™ as a vaccine against COVID-19. Vaccine effectiveness was defined in our study as the number of HCWs who got protected from contracting an infection following vaccination divided by the total number of HCWs who had received the vaccine [i.e., (Total HCWs - HCWs who developed breakthrough infection)/Total HCWs]. This was reported as a percentage and was compared between the two vaccines being studied.
We performed an extensive literature review using PUBMED, WHO guidelines on COVID, and other databases searching the keywords COVID-19, SARS Cov-2, vaccination, HCWs, Covishield, Covaxin, breakthrough infection, and efficiency. The lacunae in knowledge were identified regarding postvaccine infection in HCWs. Based on our literature review and opinion of stakeholders (HCWs with at least 5 years of experience), a mix of open-ended and multiple-choice questions were framed and then discussed among the expert group. The language of the survey questions was set as simple as possible for easy understanding of everyone including participants like nursing and technical staff. Feedback was sought from technicians and nurses regarding the language, simplicity, and ease of understanding of the survey. The final questionnaire [Annexure 1] consisted of a set of 29 open-ended and multiple-choice questions which were sent out for validation to a panel of 10 medical professionals from diverse medical specialties. Seven of them responded to our request and graded it as per the instructions laid down. There was a consensus among all seven panelists regarding the structure and content of the self-structured questionnaire. The mean of item-wise content validity index for relevance, simplicity, clarity, and ambiguity was 0.96, 0.95, 0.99, and 0.90 respectively.
The Kappa statistic for each question (for relevance, simplicity, clarity, and ambiguity) was found to be between 0.75 and 1 in our study, which is considered excellent as per the strength of agreement categorized by Cicchetti.[6] The content validity index of the overall scale (S-CVI) is the proportion of individual items which had been given a rating of “very relevant” by all the experts involved. The I-CVI was found to be 0.8, while S-CVI/average was 0.9, which again fully satisfied the validation criteria.[7] The suggestions of these experts were incorporated into the final questionnaire, which was then prepared using Google forms, with options of filling the applicable sections. The link to the survey was circulated via e-mails in August 2021. A snowball method of sampling technique was used to disseminate the survey widely across India, and the respondents were requested to forward the link via email further (with all the emails sent further to be carbon-copied (“cc”) to us so that response rate could be known). The HCWs in India comprising of doctors in clinical, preclinical or paraclinical branches, nursing, technical, and support staff who received at least one of the two doses of Covaxin® or Covishield™, irrespective of the previous history of infection and were willing to participate in the study were included.
HCWs in India who did not receive any dose of Covaxin®/Covishield™ and HCWs who were not conversant with basic English language were excluded. A brief description of the survey was given before participation in the survey and consent was then obtained for voluntary participation. After they consented to take the survey, a set of several mandatory questions appeared sequentially which the participants had to answer. Nonresponders were sent reminders twice a week till the desired sample size was attained (3 weeks), after which the survey was closed and total responses received were pooled, charted, sorted, collated, and evaluated. Anonymity regarding the subject’s or the institute’s identity was maintained throughout data collection and analysis and the results were kept confidential.
Statistical analysis
Based on a pilot study done by us on 50 HCWs who were vaccinated with either Covishield or Covaxin, with or without previous history of COVID-19 infection, the infection rate (proportion of HCWs with infection) was 23%. Taking this value as reference, the sample size with a 2.5% margin of error and 5% level of significance was 1089 HCWs, with a two-sided alpha error of 5%. After eliminating incomplete or double responses, we finally got 1096 responses that were analyzed.
The categorical variables are presented in the form of numbers and percentages (%). The association of the qualitative variables was analyzed using the Chi-square test. A P -value of less than 0.05 was considered significant. If any cell had an expected value of < 5, then Fisher’s exact test was used. The data entry was done in the Microsoft EXCEL spreadsheet and the final analysis was done with the use of Statistical Package for Social Sciences software version 21.0 (IBM Inc., Chicago, USA). For statistical significance, a P value of less than 0.05 was considered significant.
Results
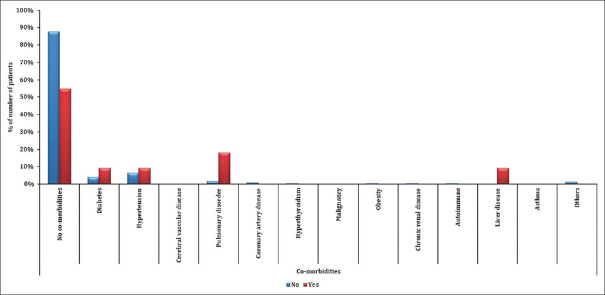
This Google form-based survey was circulated via emails to 2000 HCWs, and we received 1104 responses out of which 1096 were complete, with a response rate of 54.8%. Out of all the HCWs on the frontline, most respondents were doctors 86.4% (64.6% clinical specialities, 21.8% in pre/paraclinical specialities), 5.6% nursing staff, and 8.6% were technical staff and support staff. The respondents were from different states of India and 54.93% were females and 44.62% respondents were males. Among the respondents, 47.9% were in the 20–30-year age group, 31.2% in the 30–40 age group, 11.59% in the 40–50-year age group, 5.84% were between 50 and 60 years, and 3.38% were above 60 years of age. Most of our respondents worked in government medical colleges, 32.57% in private hospitals, and the rest were in either government nonteaching institutes or had a private practice. Among the subjects, 85.13% had no comorbidities and systemic illnesses, whereas 6.11% had hypertension, and 4.56% had diabetes mellitus, while the rest had other systemic illnesses like pulmonary disorders, cerebral vascular disease, coronary artery disease, etc., [Tables 1 and 2]
Table 1.
Distribution of sociodemographic characteristics of study subjects
| Sociodemographic characteristics | Frequency | Percentage |
|---|---|---|
| Age (years) | ||
| 20-30 | 525 | 47.90 |
| 30-40 | 342 | 31.20 |
| 40-50 | 127 | 11.59 |
| 30-40 | 1 | 0.09 |
| 50-60 | 64 | 5.84 |
| >60 | 37 | 3.38 |
| Gender | ||
| Female | 602 | 54.93 |
| Male | 489 | 44.62 |
| Prefer not to say | 5 | 0.46 |
| Designation | ||
| Doctor (pre/paraclinical) | 231 | 21.08 |
| Doctor (clinical speciality) | 708 | 64.60 |
| Nursing staff | 62 | 5.66 |
| Technical staff | 35 | 3.19 |
| Other support staff | 60 | 5.47 |
| State | ||
| Andhra Pradesh | 23 | 2.10 |
| Arunachal Pradesh | 2 | 0.18 |
| Assam | 20 | 1.82 |
| Bihar | 16 | 1.46 |
| Chandigarh | 11 | 1.00 |
| Chhattisgarh | 30 | 2.74 |
| Delhi | 298 | 27.19 |
| Gujarat | 51 | 4.65 |
| Haryana | 62 | 5.66 |
| Himachal Pradesh | 40 | 3.65 |
| Jammu and Kashmir | 57 | 5.20 |
| Jharkhand | 5 | 0.46 |
| Karnataka | 55 | 5.02 |
| Kerala | 29 | 2.65 |
| Madhya Pradesh | 15 | 1.37 |
| Maharashtra | 45 | 4.11 |
| Manipur | 1 | 0.09 |
| Meghalaya | 1 | 0.09 |
| Mizoram | 3 | 0.27 |
| Odisha | 3 | 0.27 |
| Puducherry | 3 | 0.27 |
| Punjab | 20 | 1.82 |
| Rajasthan | 58 | 5.29 |
| Tamilnadu | 139 | 12.68 |
| Telangana | 14 | 1.28 |
| Tripura | 4 | 0.36 |
| Uttar Pradesh | 67 | 6.11 |
| Uttarakhand | 5 | 0.46 |
| West Bengal | 19 | 1.73 |
| Set up of work | ||
| Government medical college | 596 | 54.38 |
| Government nonteaching institution | 86 | 7.85 |
| Private hospital | 357 | 32.57 |
| Freelancer | 57 | 5.20 |
| Comorbidities | Frequency | Percentage |
| No comorbidities | 933 | 85.13 |
| Diabetes | 50 | 4.56 |
| Hypertension | 67 | 6.11 |
| Cerebral vascular disease | 2 | 0.18 |
| Pulmonary disorder | 24 | 2.19 |
| Coronary artery disease | 9 | 0.82% |
| Hyperthyroidism | 5 | 0.46% |
| Malignancy | 1 | 0.09% |
| Obesity | 3 | 0.27% |
| Chronic renal disease | 3 | 0.27% |
| Autoimmune | 1 | 0.09% |
| Liver disease | 1 | 0.09% |
| Asthma | 1 | 0.09% |
| Others | 23 | 2.10% |
Table 2.
Association of sociodemographic characteristics with severity of infection
| Sociodemographic characteristics | Mild disease (n=235) | Moderate disease (n=19) | Severe disease (n=2) | Total | P |
|---|---|---|---|---|---|
| Age (years) | |||||
| 20-30 | 104 (44.26%) | 4 (21.05%) | 0 (0%) | 108 (42.19%) | 0.017@ |
| 30-40 | 79 (33.62%) | 4 (21.05%) | 1 (50%) | 84 (32.81%) | |
| 40-50 | 29 (12.34%) | 6 (31.58%) | 1 (50%) | 36 (14.06%) | |
| 30-40 | 1 (0.43%) | 0 (0%) | 0 (0%) | 1 (0.39%) | |
| 50-60 | 16 (6.81%) | 3 (15.79%) | 0 (0%) | 19 (7.42%) | |
| >60 | 6 (2.55%) | 2 (10.53%) | 0 (0%) | 8 (3.13%) | |
| Gender | |||||
| Female | 136 (57.87%) | 7 (36.84%) | 1 (50%) | 144 (56.25%) | 0.137@ |
| Male | 99 (42.13%) | 12 (63.16%) | 1 (50%) | 112 (43.75%) | |
| Designation | |||||
| Doctor (pre/paraclinical) | 48 (20.43%) | 0 (0%) | 1 (50%) | 49 (19.14%) | 0.0005@ |
| Doctor (clinical speciality) | 155 (65.96%) | 10 (52.63%) | 0 (0%) | 165 (64.45%) | |
| Nursing staff | 14 (5.96%) | 5 (26.32%) | 1 (50%) | 20 (7.81%) | |
| Technical staff | 7 (2.98%) | 1 (5.26%) | 0 (0%) | 8 (3.13%) | |
| Other support staff | 11 (4.68%) | 3 (15.79%) | 0 (0%) | 14 (5.47%) | |
| State | |||||
| Andhra Pradesh | 3 (1.28%) | 0 (0%) | 0 (0%) | 3 (1.17%) | 0.939@ |
| Arunachal Pradesh | 1 (0.43%) | 0 (0%) | 0 (0%) | 1 (0.39%) | |
| Assam | 1 (0.43%) | 0 (0%) | 0 (0%) | 1 (0.39%) | |
| Bihar | 3 (1.28%) | 1 (5.26%) | 0 (0%) | 4 (1.56%) | |
| Chandigarh | 1 (0.43%) | 0 (0%) | 0 (0%) | 1 (0.39%) | |
| Chhattisgarh | 9 (3.83%) | 0 (0%) | 0 (0%) | 9 (3.52%) | |
| Delhi | 96 (40.85%) | 9 (47.37%) | 1 (50%) | 106 (41.41%) | |
| Gujarat | 9 (3.83%) | 1 (5.26%) | 0 (0%) | 10 (3.91%) | |
| Haryana | 15 (6.38%) | 0 (0%) | 0 (0%) | 15 (5.86%) | |
| Himachal Pradesh | 4 (1.70%) | 0 (0%) | 0 (0%) | 4 (1.56%) | |
| Jammu and Kashmir | 12 (5.11%) | 1 (5.26%) | 1 (50%) | 14 (5.47%) | |
| Jharkhand | 3 (1.28%) | 0 (0%) | 0 (0%) | 3 (1.17%) | |
| Karnataka | 3 (1.28%) | 0 (0%) | 0 (0%) | 3 (1.17%) | |
| Kerala | 5 (2.13%) | 1 (5.26%) | 0 (0%) | 6 (2.34%) | |
| Madhya Pradesh | 5 (2.13%) | 0 (0%) | 0 (0%) | 5 (1.95%) | |
| Maharashtra | 4 (1.70%) | 1 (5.26%) | 0 (0%) | 5 (1.95%) | |
| Mizoram | 1 (0.43%) | 0 (0%) | 0 (0%) | 1 (0.39%) | |
| Odisha | 1 (0.43%) | 0 (0%) | 0 (0%) | 1 (0.39%) | |
| Punjab | 4 (1.70%) | 0 (0%) | 0 (0%) | 4 (1.56%) | |
| Rajasthan | 13 (5.53%) | 1 (5.26%) | 0 (0%) | 14 (5.47%) | |
| Tamilnadu | 17 (7.23%) | 1 (5.26%) | 0 (0%) | 18 (7.03%) | |
| Telangana | 4 (1.70%) | 0 (0%) | 0 (0%) | 4 (1.56%) | |
| Tripura | 1 (0.43%) | 0 (0%) | 0 (0%) | 1 (0.39%) | |
| Uttar Pradesh | 14 (5.96%) | 3 (15.79%) | 0 (0%) | 17 (6.64%) | |
| Uttarakhand | 2 (0.85%) | 0 (0%) | 0 (0%) | 2 (0.78%) | |
| West Bengal | 4 (1.70%) | 0 (0%) | 0 (0%) | 4 (1.56%) | |
| Set up of work | |||||
| Government medical college | 135 (57.45%) | 12 (63.16%) | 1 (50%) | 148 (57.81%) | 0.641@ |
| Government nonteaching institution | 20 (8.51%) | 0 (0%) | 0 (0%) | 20 (7.81%) | |
| Private Hospital | 67 (28.51%) | 5 (26.32%) | 1 (50%) | 73 (28.52%) | |
| Freelancer | 13 (5.53%) | 2 (10.53%) | 0 (0%) | 15 (5.86%) |
@ Fisher’s exact test
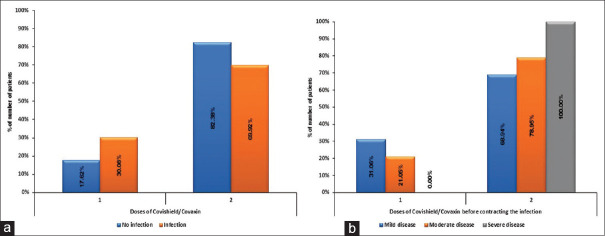
Out of 1096 HCWs who received either one or two doses of Covaxin® or Covishield™ as a vaccine against COVID-19, 23.36% (n = 256/1096) had a breakthrough infection. Out of 23.36% who had a breakthrough infection, 7% (78/1096) had received one dose and 16.3% (178/1096) had received two doses of the respective vaccines. Out of all vaccinated HCWs, 0.8% had a severe disease where severity was defined as per AIIMS guideline of having respiratory rate >30 or room air saturation <90% (described in our survey).[8] While 91% of those infected despites being vaccinated had mild disease, 8.2% had a moderate disease. A total of 3 HCWs required ICU care (1.17% of infected HCWs) and 11 required supplemental oxygen (4.3%) [Figure 1].
Figure 1.
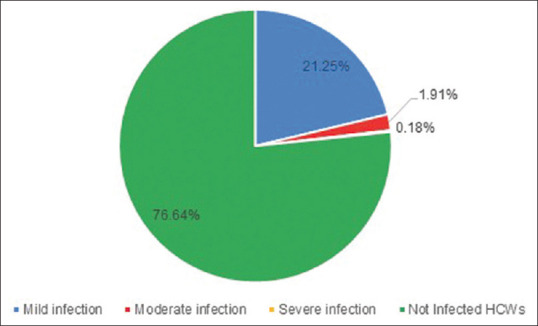
Percentage of infected and noninfected HCWs postvaccination
Sixty-eight percent (68.16%) of all the vaccinated HCWs received Covishield™, while the rest received Covaxin®. Correspondingly, out of all the vaccinated HCWs who had a breakthrough infection (n = 256), 62.50% had received Covishield™(n = 160) and 37.5% Covaxin® (n = 96), respectively [Figure 2]. The breakthrough infection prevalence was less with Covishield™ (160/747; 21.41%) when compared to Covaxin® (96/349; 31.84%). The point estimate for effectiveness in preventing infection was significantly better with Covishield™ (78.5% vs. 72.4%) (P = 0.0260). More percentage of participants reported getting infected from hospital areas (78.52%) as compared to outside the hospital settings (21.48%). Attaining positivity while working in COVID care areas like COVID ICU/ward and COVID suspect area was found to be lesser (26.95%). At the time of viral exposure, only 40.23% of HCWs were wearing personal protective equipment (PPE), while the rest of them (59.77%) were not wearing PPE. Gender, type of setup, and state of practice had no major association with severity of COVID-19 infection, rate of hospitalization, or requirement of supplemental oxygen. The severity of infection was more in the age group of 30–50 years (P = 0.0170) as compared to other age groups. The doctors belonging to clinical branches had more severe diseases than pre/paraclinical medical professionals and other categories of HCWs (P = 0.0005).
Figure 2.
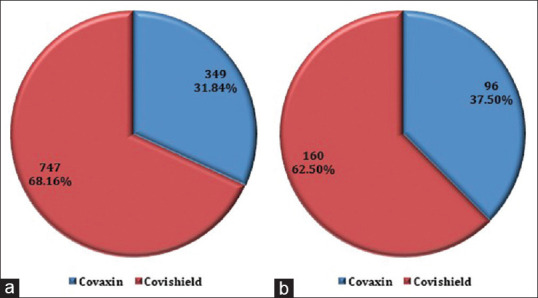
(a) Distribution of vaccine given to all healthcare workers; (b) Distribution of vaccine given in infected HCWs
HCWs with preexisting pulmonary disorders had a statistically significant association for requiring supplemental oxygenation (P = 0.0230), for hospitalization in ICU (P = 0.0007), and severity of infection (P = 0.02). Liver disease was also associated with need for a higher degree of supplemental oxygen (P = 0.0250) [Figure 3].
Figure 3.
Association of comorbidities with required supplemental oxygen
Seventy percent (n = 178) of all breakthrough infections occurred after the second dose (62.5% with Covaxin® and 74.3% with Covishield™) [Figure 4]. Seventy-eight out of two hundred twenty-five HCWs got infected after one dose of vaccine (34.66%). (where 225 = total HCWs who received one dose of vaccine)
Figure 4.
Association of doses of Covishield/Covaxin with infection (a) and its severity (b)
One hundred seventy-eight out of eight hundred seventy-one got infected after two doses of vaccine (20.43%) (871 = total number of HCWs who received two doses of vaccine). So, the prevalence of HCWs who got infected with COVID-19 is higher after the first dose 34.66% as compared to after second dose (20.43%). Among all the HCWs vaccinated who got infected, 93.65% (Covaxin®, n = 89 and Covishield™, n = 150) had a perception that vaccination helped decrease the severity of infection in them. Nearly all those who were infected after vaccination (97.85%, n = 150) were in favor of encouraging the public to get vaccinated against COVID-19.
Discussion
The present study investigated the prevalence of SARS-CoV-2 in the vaccinated HCWs including doctors (clinical speciality and pre/paraclinical), nursing staff, technical staff, and support staff who had received at least one dose of the respective vaccines (either Covaxin® or Covishield™). We targeted HCWs only as the risk of infection remains highest in this population and it was more feasible due to convenience sampling.
The study findings revealed that Covishield™ showed a significantly higher point estimate of effectiveness in preventing infection as compared to Covaxin® (78.5% vs 72.4%). The prevalence of infection was more in individuals who had received one dose as compared to both the doses. Increased severity of the disease was seen in young HCWs, those with preexisting comorbidities and those belonging to clinical branches.
Various vaccines are available across the world against COVID-19 with varying efficacy like Pfizer-BioNTech (BNT162b2), Moderna (mRNA-1273), Johnson and Johnson, Oxford-AstraZeneca, etc., In India, Covaxin® (BBV152) and Covishield™ (AZD1222 or ChAdO × 1-S) are approved by the Drug Controller General of India for restricted use in an emergency.[9] For Covaxin® (BBV152), the interim results showed the efficacy of 81% in preventing infection in those who have received both doses and not been previously infected by SARS-Cov-2 and 100% efficacy in preventing severe disease if infected.[10,11] Covishield™ (AZD1222 or ChAdOx1-S) showed a 71% efficacy in preventing infection.[12] Data evaluating the effect of vaccination on occurrence of breakthrough infections, decreased transmission, and disease morbidity is sparse at present.
HCWs in most of the countries including India have been a high-risk population for COVID-19 infection and have been disproportionately more affected by COVID-19.[13,14] So, HCWs were prioritized for COVID-19 vaccination before the general public starting January 2021.[15] In our study, nearly one out of four HCWs (23.36%) reported being infected with COVID-19 during the second wave either as a positive RTPCR report, CT scan findings suggestive of COVID-19 infection, or symptoms after exposure to laboratory-confirmed COVID-19 positive patients. Breakthrough infections after vaccination have been reported in other studies as well with varying prevalence. In India, studies report varying incidence/prevalence from 1.6% by Rana et al.,[16] 16.9% by Tyagi et al.,[17], and 11.94% by Goenka et al.[18] The point prevalence in our study (23.36%) was higher since we conducted the survey in a single high-risk population of HCWs, whereas the majority studies had been conducted as a population-based survey or single center incidence studies. In a study by Tyagi et al.[17], 123 employees in a single diabetes center were studied and 16.9% incidence of breakthrough infection was reported after one or two doses of vaccine. Furthermore, we conducted the study once the second wave had just subsided; hence, peak exposure of the HCWs had already happened. The Centre for Disease Control in the USA had also reported breakthrough infections. A study by Hacisuleyman et al.[19] reported an incidence of breakthrough infection of 0.5% after HCWs received two doses of either Pfizer-BioNTech or Moderna vaccine. Similarly, in the UK, the SIREN study showed decreased the incidence of infection postvaccination.[20] Seven percent (78/1096) of all vaccinated HCWs got infected after receiving only one dose of either Covaxin® or Covishield™, whereas 16.3% (256/1096) had received both doses of either vaccine before they were infected. The probable reasons for a breakthrough infection may be that people who were vaccinated with either Covaxin® or Covishield™ have shown almost 55% fewer antibodies against B.1.617 signature mutations delta variant as compared to those generated against B.1 variant.[3,21,22] It may be possible that the person contracted the infection just before the second dose.[23,24] Due to the surge in pandemics during the months of April–May, the point prevalence of infected HCWs in our study was high.[25] Majority of HCWs contracted the disease while working in hospital, and this shows that hospital-acquired infection rates are higher than the incidence in the community.[26] As a corollary, HCWs who were working in known COVID-19 care areas at the time of infection contracted the disease to a lesser extent as compared to non-COVID-19 which may be due to the protection offered by the use of PPE in known COVID-19 areas.
Data from different countries suggests that 14–19% of COVID-19-infected patients are hospitalized, and 3–5% develop severe disease requiring intensive care.[27,28,29] In our study, the breakthrough infection HCWs was mild in 91%, moderate in 8.2%, and only 0.8% had severe disease.[8] Among the infected, 4% required supplemental oxygen and 1.17% needed ICU care, suggesting that vaccination decreased the severity of infection in HCWs. Vaccination decreased the severity of breakthrough infection in other countries also.[26] Moreover, PPE compliance was protective in our study because approximately 60% of HCWs who got the breakthrough infection were not wearing PPE at the time of exposure.
In our study, the severity of infection was more in the younger HCWs (30–50 years) (P = 0.0170) probably because most of them were frontline workers during the peak of pandemic. Many had preexisting comorbidities which increased the severity of infection because of various systemic implications of the disease are known to complicate COVID-19 infection.[30] Doctors in clinical branches experienced more breakthrough infections than other branches possibly due to the increased viral exposure in clinical areas.
Maintaining high vaccination coverage among staff members is also important to reduce opportunities for transmission within hospitals. Moreover, HCWs should continue to follow the recommended infection prevention and control practices regardless of vaccination status. Strengths of the study include that the survey of breakthrough infection postvaccination was conducted on a nationwide level and included a large sample of all categories of HCWs to improve the generalizability of the study. Data collection was done after India had just experienced the peak of the second wave and HCWs had high chances of exposure to the virus.
Our study had certain limitations. First, deaths among HCW due to COVID-19 have not been taken into consideration in this data. Though it would have been useful information, the survey was a self-administered questionnaire, and it would therefore not have been possible to get this data through the medium of this survey. Second, we did not conduct any laboratory investigation like RTPCR/CT scan/serological tests or collect any medical records for the survey; the data is based on the credibility of the responses by participants and their self-reporting. However, since complete anonymity was maintained, the data collected are expected to be reliable. Third, we did not capture the duration between the dose of vaccine and getting the infection, and this could affect the study outcomes (albeit minimally considering the sample size). Further, recall bias could be a hindrance, but vaccination had started in January end, so the survey was <6 months from the start of vaccination and recall within this period should not be an issue. Lastly, we had designed the survey in simple English only, but a lot of support staff may not be able to complete the survey due to a lack of understanding of English language.
To conclude, our data indicate that though breakthrough infection after vaccination does occur, possibility of contracting a severe infection is very low. Covishield™ performed significantly better than Covaxin® in terms of effectiveness against the disease. Clinical branches were found to have a higher risk and those with comorbidities had higher severity of the disease. We, therefore, reiterate that these high-risk subgroups should be encouraged to undergo vaccination at the earliest, and despite vaccination, there remains a pressing need to maintain the layers of mitigation strategies such as masking and social distancing.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ANNEXURE
A nationwide survey on the epidemiology, type of vaccine and risk factors in Healthcare Workers contracting COVID 19 infection postvaccination
Greetings!
We are a team of doctors from Safdarjung Hospital and AIIMS in New Delhi conducting this study.
First of all, a big thank you to all of you for being a frontline warrior against COVID 19 for more than a year now. Hope you are all keeping safe. I would also like to congratulate you on getting vaccinated.
We are conducting this survey to find out the incidence of breakthrough infections after vaccination against COVID 19, as well as the severity of infection in vaccinated individuals and the risk factors involved. We hope that this data will help us encourage the general public to get themselves vaccinated. Those who have not yet been vaccinated are not eligible to take up the survey. The survey is in the English language and those with problems reading or understanding it can skip the survey.
Institutional Ethics approval was sought before starting the survey. The participation is purely voluntary, and if you chose to not participate, you can leave the page. Your name, identity, institute or other personal details are not asked in the questionnaire; thus, your anonymity will be upheld. Only your email ID is asked for recording the response rate. Your answers will not be revealed and blinding is done to keep your email ID hidden during analysis. This will take 1-2 min of your time.
Thanks in advance.
*Required
1. Email *..........................................
General information
2. Consent to participate: I have reviewed the Participation Information Sheet provided and understand that my participation is voluntary. By clicking on the "I agree" button, I hereby give my consent to be part of the study. * (Tick all that apply.)
□ I agree
3.What is your age (in years)? *
□ 20-30
□ 30-40
□ 40-50
□ 50-60
□ >60
4.What is your gender? *
□ Male
□ Female
□ Prefer not to say
5.Among the front line warriors, what is your designation? *
□ Doctor (pre/paraclinical)
□ Doctor (clinical speciality)
□ Nursing staff
□ Technical staff
□ Other support staff
6.Which state of India do you belong to? *
7.Do you have any systemic comorbidities? *
□ Yes
□ No
8.If you have any comorbidities, please specify: (can choose more than 1)
□ Diabetes
□ Hypertension
□ Coronary artery disease
□ Pulmonary disorder
□ Chronic renal disease
□ Cerebral vascular disease
□ Liver disease
□ Other:..........................
9.What type of setup you are working? *
□ Government medical college
□ Government nonteaching institution
□ Private hospital
□ Freelancer
10.Did you get infected with COVID 19 despite taking the vaccine? (Infection: RTPCR positive/CT suggestive of COVID infection/symptoms after contact with lab positive case of COVID) *
□ Yes Skip to question 11
□ No Skip to question 28
□ For individuals who took the vaccine and got infected with COVID:
11.Where were you posted when you contracted the infection? *
□ COVID ICU
□ COVID ward
□ Suspect ICU/ward
□ Non COVID area
□ Outside hospital setting
12.Were you wearing personal protective equipment (PPE) when you contracted the infection?*
□ Yes
□ No
13.Which vaccine did you take? *
□ Covaxin Skip to question 14
□ Covishield Skip to question 21
□ Skip to question 14
□ HCWs infected with COVID after getting vaccinated with Covaxin:
14.How many doses of Covaxin did you get before contracting the infection? *
□ 1
□ 2
15.If you got the second dose, did 2 weeks pass after the second dose?
□ Yes
□ No
16.What was the severity of your infection? *

□ Mild disease
□ Moderate disease
□ Severe disease
17.Did you require supplemental oxygen? *
□ Yes
□ No
18.Did you require hospitalization? *
□ Tick all that apply.
□ No
□ In the ward In the ICU
19.Despite getting the infection, will you encourage/advise vaccination to the general public? *
□ Tick all that apply.
□ Yes
□ No
20.Do you think vaccination decreased the severity of the disease you got as compared to individuals who were not vaccinated? *
□ Tick all that apply.
□ Yes
□ No
HCWs infected with COVID after getting vaccinated with Covishield:
21.How many doses of Covishield did you get? *
Tick all that apply.
□ 1
□ 2
22.If you got the second dose, did 2 weeks pass after the second dose?
□ Yes
□ No
23.What was the severity of your infection? *

□ Mild disease
□ Moderate disease
□ Severe disease
24.Did you require supplemental oxygen? *
□ Yes
□ No
25.Did you require hospitalization? *
□ No
□ In ward
□ In ICU
26.Despite getting the infection, will you encourage/advise vaccination to the general public? *
□ Yes
□ No
27.Do you think vaccination decreased the severity of the disease you got as compared to individuals who were not vaccinated? *
□ Yes
□ No
HCWs who got Thank you for your response. We hope you and your family are doing vaccinated but did not well and you continue to remain safe through this pandemic. Regards. get infection:
28.Did you get both doses of the vaccine? *
□ Yes
□ No
29.What vaccine did you get? *
□ Covaxin
□ Covishield
References
- 1.Center for Disease Control and Prevention. 2009 H1N1 Pandemic. 2021. [Last assessed on 2021 Sep 15]. Available from: https://www.cdc.gov/flu/pandemic-resources/2009-h1n1-pandemic.html .
- 2.Worldometer Corona virus death toll. [Last accessed on 2021 Dec 11]. Available from: https://www.worldometers.info/coronavirus/coronavirus-death-toll/
- 3.Ella R, Reddy S, Blackwelder W, Potdar V, Yadav P, Sarangi V, et al. Efficacy, safety, and a lot to lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): A double-blind, randomised, controlled phase 3 trial. bioRxiv. 2021 doi: 10.1016/S0140-6736(21)02000-6. doi: org/10.1101/2021.06.30.21259439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–93. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohfw.Gov.in. Vaccination status. [Last updated on 2021 Dec 10; Last accessed on 2021 Dec 10]. Available from: https://www.mohfw.gov.in/
- 6.Cichetti DV. On a model for assessing the security of infantile attachment: Issues of observer reliability and validity. Behav Brain Sci. 1984;7:149–50. [Google Scholar]
- 7.Lynn MR. Determination and quantification of content validity. Nurs Res. 1986;33:382–5. [PubMed] [Google Scholar]
- 8.Clinical Management Protocol for COVID-19. [updated 2021 May 19; Last accessed on 2021 Aug 19]. Available from: https://www.mohfw.gov.in/pdf/COVID19 Clinical Management Protocol AlgorithmAdults19thMay2021.pdf .
- 9.Press Statement by the Drugs Controller General of India (DCGI) on Restricted Emergency approval of COVID-19 virus vaccine. Gov. in. January 3rd. [Last accessed on 2021 Aug 02]. Available from: https://www.icmr.gov.in/pdf/press_realease_files/HFW_DCGI_energency_use_authorisation_03012021_2.pdf .
- 10.Ella R, Vadrevu KM, Jogdand H, Prasad S, Reddy S, Sarangi V, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: A double-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;21:637–46. doi: 10.1016/S1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ella R, Reddy S, Blackwelder W, Potdar V, Yadav P, Sarangi V, et al. Efficacy, safety, and lot to lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): A, double-blind, randomised, controlled phase 3 trial. medRxiv. 2021 doi: 10.1016/S0140-6736(21)02000-6. doi: https://doi.org/10.1101/2021.06.300.21259439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mutambudzi M, Niedwiedz C, Macdonald EB, Leyland A, Mair F, Anderson J, et al. Occupation and risk of severe COVID-19: A prospective cohort study of 120 075 UK Biobank participants. Occup Environ Med. 2020;78:307–14. doi: 10.1136/oemed-2020-106731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen LH, Drew DA, Graham MS, Joshi AD, Guo CG, Ma W, et al. Risk of COVID-19 among front-line health-care workers and the general community: A prospective cohort study. Lancet Public Health. 2020;5:e475–83. doi: 10.1016/S2468-2667(20)30164-X. doi: 10.1016/S2468-2667 (20) 30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Hindu Business Line. Our Bureau. India’s Covid-19 vaccine campaign off to a roaring start. 2021. Available from: https://www.thehindubusinessline.com/news/national/indias-vaccines-enjoy-global-credibility-because-of-their-track-record-pm-modi/article33585938.ece .
- 16.Rana K, Mohindra R, Pinnaka L. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;385:e7. doi: 10.1056/NEJMc2107808. doi: 10.1056/NEJMc2107808. [DOI] [PubMed] [Google Scholar]
- 17.Tyagi K, Ghosh A, Nair D, Dutta K, Bhandari PS, Ansari IA, et al. Breakthrough COVID19 infections after vaccinations in healthcare and other workers in a chronic care medical facility in New Delhi, India. Diabetes Metab Syndr. 2021;15:1007–8. doi: 10.1016/j.dsx.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goenka M, Afzalpurkar S, Goenka U, Das SS, Mukherjee M, Jajodia S, et al. Seroprevalence of COVID-19 amongst health care workers in a tertiary care hospital of a metropolitan city from India. J Assoc Physicians India. 2020;68:14–9. [PubMed] [Google Scholar]
- 19.Hacisuleyman E, Hale C, Saito Y, Blachere NE, Bergh M, Conlon EG, et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;384:2212–8. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): A prospective, multicentre, cohort study. Lancet. 2021;397:1725–35. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wall EC, Wu M, Harvey R, Kelly G, Warchal S, Sawyer C, et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397:2331–3. doi: 10.1016/S0140-6736(21)01290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh UB, Rophina M, Chaudhry DR, Senthivel V, Bala K, Bhoyar RC, et al. Genomic analysis of symptomatic SARS-CoV-2 vaccine breakthrough infections from a tertiary care centre in India. OSF Preprints; 2021. [Last accessed on 2021 Sep 20]. Available from: https://osf.io/fgd4x/
- 23.Keehner J, Horton LE, Pfeffer MA, Longhurst CA, Schooley RT, Currier JS, et al. SARS-CoV-2 infection after vaccination in health care workers in California. N Engl J Med. 2021;384:1774–5. doi: 10.1056/NEJMc2101927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kustin T, Harel N, Finkel U, Perchik S, Harari S, Tahor M, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2 mRNA vaccinated individuals. bioRxiv. 2021 doi: 10.1038/s41591-021-01413-7. doi: 10.1101/2021.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delhi reports more Covid cases and deaths in April-May than since the beginning of the pandemic. News 18. Available from: https://www.news18.com/news/india/delhi-reportsmore-covid-cases-deaths-in-april-may-than-since-the-beginning-of-pandemic-3751346.html)
- 26.Richterman A, Meyerowitz EA, Cevik M. Hospital-acquired SARS-CoV-2 infection: Lessons for public health. JAMA. 2020;324:2155–6. doi: 10.1001/jama.2020.21399. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Huang DQ, Zou B, Yang H, Hui WZ, Rui F, et al. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2021;93:1449–58. doi: 10.1002/jmv.26424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: Prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y, Yang Q, Chi J, Dong B, Lv W, Shen L, et al. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta-analysis. Int J Infect Dis. 2020;99:47–56. doi: 10.1016/j.ijid.2020.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]