Figure 1. Strategies to harness carbene reactivity.
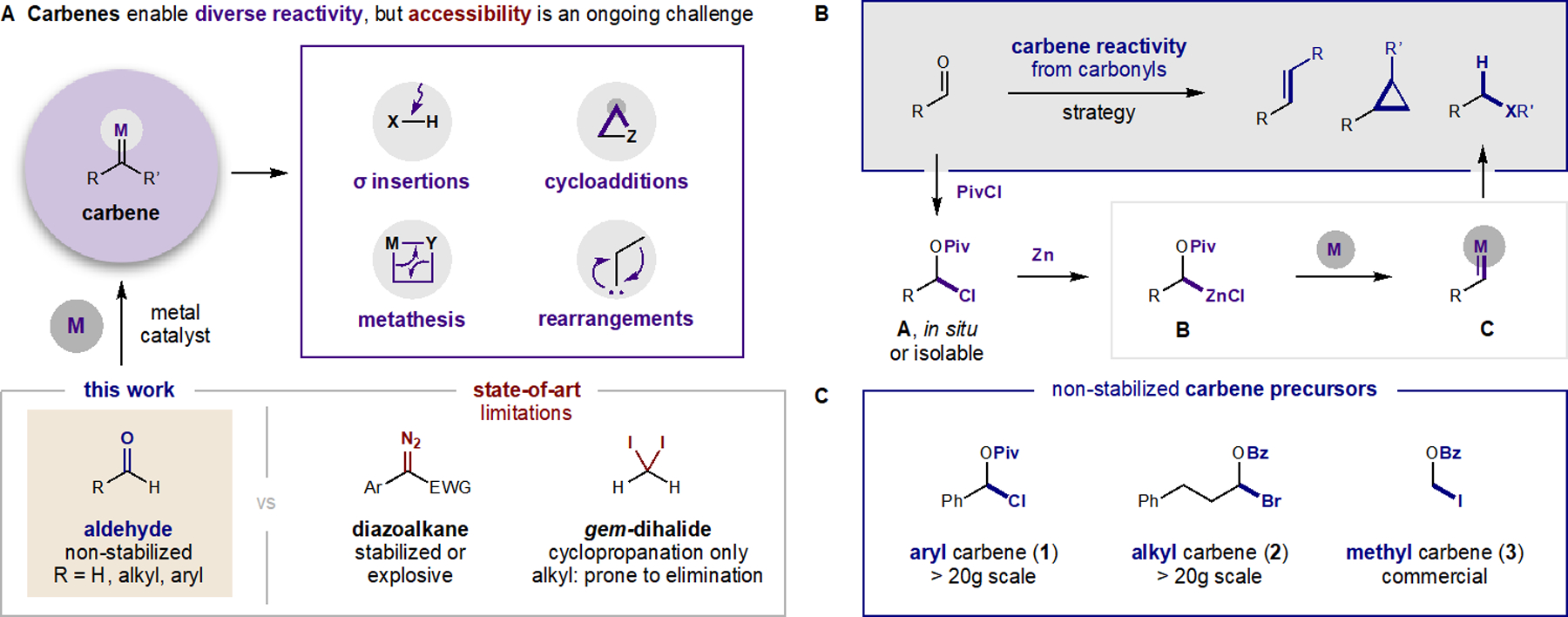
(A) Synthetic approaches to carbenes either rely on loss of N2 from diazoalkanes or reduction of gem-dihalides. Both reagent classes exhibit limited scope, stability, and reactivity in absence of aryl or α-carbonyl stabilization. (B) Our design converts carbonyls to non-stabilized carbenes via Zn insertion into α-acyloxy halides and activation by Earth-abundant metal catalysts. (C) Three classes of carbene precursors are readily prepared and exhibit significantly improved safety profiles versus diazo reagents.
