Figure 4. Mechanistic experiments.
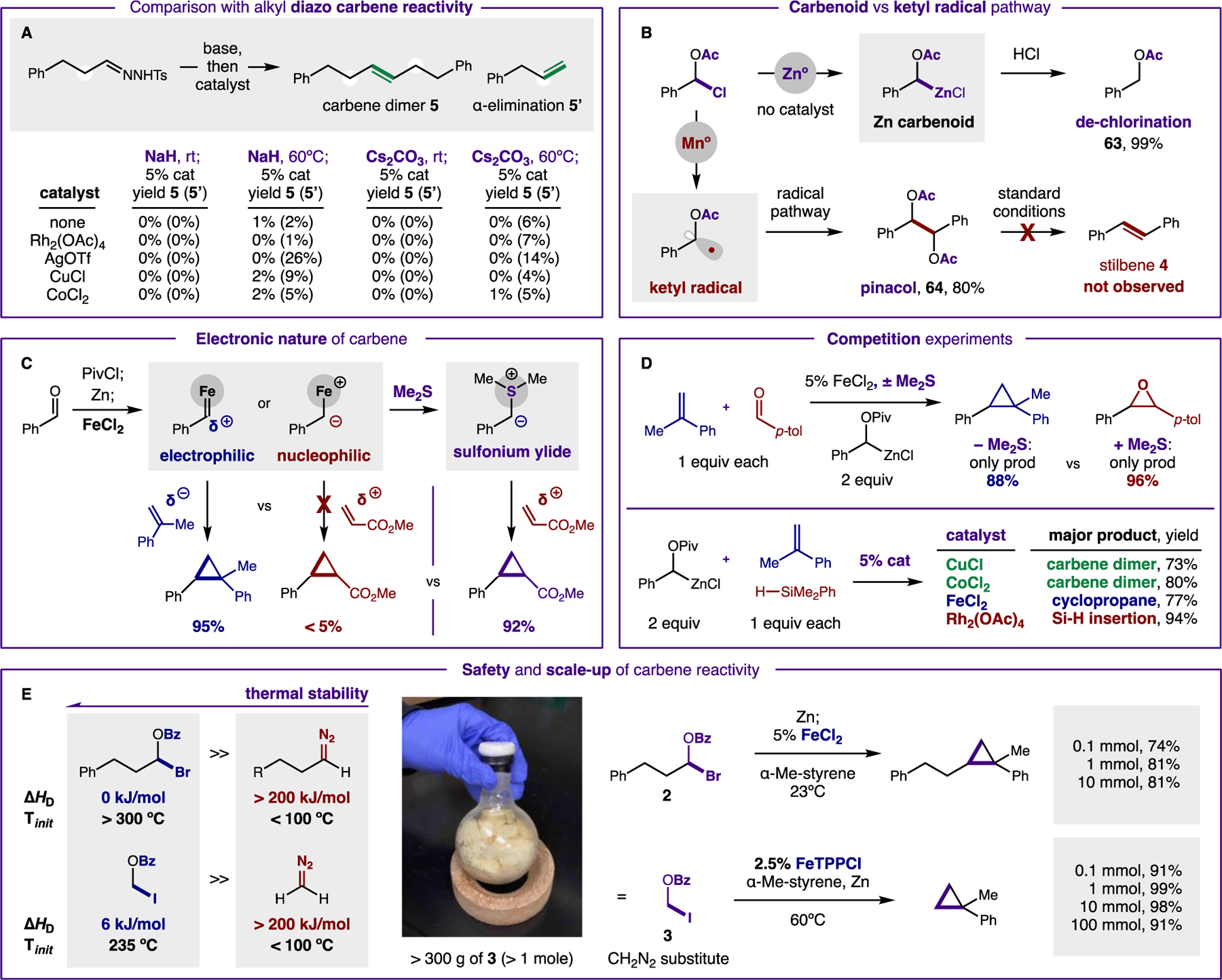
(A) This carbene reactivity is not accessible via diazoalkanes. (B) The proposed Zn carbenoid is validated by an acidic quench. Conversely, single-electron reduction affords ketyl radical reactivity, but not carbene dimerization. (C) Electrophilic character of carbene is illustrated by selective reactivity with nucleophilic traps; sulfonium ylide affords polarity-reversed reactivity with electrophiles. (D) Competition experiments illustrate catalyst role in product selectivity. (E) The improved thermal stability of these carbene precursors relative to diazoalkanes allows safer scale-up of batch chemistry.
