Abstract
Background:
Strokes are common among patients with left ventricular devices (LVAD). We hypothesize that there is ongoing cerebral microvascular injury with LVAD support and aim to describe this among LVAD-implanted patients through post-mortem neuropathologic evaluation.
Methods:
We identified and reviewed medical records of LVAD patients who underwent brain autopsy between January 2006 and December 2019 at a tertiary center. Cerebral injury was defined as both gross and microscopic injuries within the intracranial space including cerebral infarct (CI), hypoxic-ischemic brain injury (HIBI), intracranial hemorrhage (ICH), and cerebral microvascular injury. Cerebral microvascular injury was defined as microscopic brain intraparenchymal or perivascular hemorrhage, perivascular hemosiderin deposition, and perivascular inflammation.
Results:
Twenty-one patients (median age=57 years, 67% male) had autopsy after LVAD support (median LVAD support=51 days). The median time from death to autopsy was 19 hours. All 21 patients had cerebral injuries and 19 (90%) patients had cerebral microvascular injuries. Fourteen patients (78%) harbored more than one type of cerebral injuries. On gross examination, 8 patients (38%) had CI, and 6 patients (29%) had ICH. On microscopic exam, 12 patients (57%) had microscopic intraparenchymal hemorrhage, 3 patients (14%) had perivascular hemorrhage, 11 patients (43%) had perivascular hemosiderin deposition, 5 patients (24%) had meningeal hemorrhage, 13 patients had chronic perivascular inflammation (62%), and 2 patients had diffuse HIBI (10%).
Conclusion:
Among patients with LVAD, there is a high prevalence of subclinical microvascular injuries and cerebral microbleeds (CMBs), which may provide some insights to the cause of frequent cerebral injury in LVAD population.
Keywords: ventricular assist device, autopsy, cerebral infarction, cerebral hemorrhage, microvascular injury, cerebral microbleeds
Introduction
Although left ventricular assist devices (LVAD) has been shown to improve overall survival and quality of life in end-stage heart failure refractory to medical treatment, stroke remains a frequent complication, causing significant morbidity and mortality1. A study using Interagency Registry for Mechanically Assisted Circulatory Support showed ~11% of patients had at least one stroke at a median follow up of 9.8 months2. Similarly, a systematic review of 11,310 patients demonstrated an overall stroke rate of 10% at a median follow-up of 112 days (0.08 events per patient year).1 The mechanism for the high rate of cerebral injury in this population is still unclear. Previous studies have identified risk factors for stroke in LVAD population including pump thrombosis3,4, bloodstream infection5–7, LVAD brand or flow-type1,8,9, previous surgery3 and co-morbid conditions such as diabetes mellitus and high blood pressure.10,11 Both pulsatile-flow and continuous-flow (CF) LVAD patients experience adverse hemodynamic effect related to significant mechanical contact bearing, and diminished arterial pulse pressure and flow respectively. As LVAD has been more frequently utilized, it is increasingly important to understand the associated hematologic and hemodynamic changes during LVAD implantation that may predispose to cerebral injury.
A study of brain magnetic resonance imaging (MRI) obtained shortly after LVAD explant showed a high prevalence of cerebral microbleeds (CMBs) (>97%) and cortical superficial siderosis (31%), significantly higher than the healthy and heart failure controls12,13. These findings were striking as this LVAD cohort had relatively young age with median age around 40 and raises the question of presence of microvascular injuries in the LVAD population. CMBs often reflect structural abnormalities of brain vasculature and perivascular collection of blood-breakdown products, commonly associated with hypertension and cerebral amyloid angiopathy. They have been considered markers of risk of symptomatic cerebral hemorrhage.14 The reason for the high prevalence of CMBs seen in LVAD patients remains unclear. We hypothesized that high frequency of stroke in LVAD patients reflect underlying microvascular changes, as seen through CMBs in the MRI study. We aimed to assess the prevalence of cerebral microvascular changes through post-mortem brain autopsy of LVAD-supported patients.
Methods
We reviewed data of 986 patients at a single tertiary care center who underwent LVAD implantation between January 2006 to December 2019. Patients that had their LVAD explanted more than 14 days before death were excluded. This study was approved by the local institutional review board. Demographics, medical history and clinical course and complications during LVAD support were collected. Medical complications included deep venous thrombosis, pulmonary embolism, infection, pump thrombosis, and significant non-intracranial bleeding (defined as an episode of bleeding that required transfusion within 24 hours of the event). Infection was further categorized into driveline, pump, pump pocket, and bloodstream infection. Pump thrombosis was defined as an evident thrombotic formation accompanied by elevated serum lactate dehydrogenase that required pump exchange or increased anticoagulation therapy.15
Cerebral injury at autopsy was defined as injuries within the cranium visible both grossly and microscopically, including cerebral infarction (CI), intracranial hemorrhage (ICH) including intraparenchymal hemorrhage (IPH), subarachnoid hemorrhage (SAH), and subdural hematoma (SDH), meningeal hemorrhage, CMBs and diffuse hypoxic-ischemic brain injury (HIBI). Diffuse HIBI was pathologically defined as global hypoxic-ischemic encephalopathy with diffuse involvement of the brain. CMBs in our study was further defined pathologically as microscopic brain intraparenchymal and perivascular hemorrhage with small foci (<1mm), and/or perivascular hemosiderin deposition. Cerebral microvascular injury was defined as microscopic brain intraparenchymal or perivascular hemorrhage, perivascular hemosiderin deposition, and perivascular inflammation. A patient was determined to have clinical findings of LVAD associated cerebral injury if the diagnosed was made prior to death including ischemic stroke (IS), ICH and global cerebral ischemia.
Neuropathological Preparation
Following a minimum of 10 days of immersion fixation in 10% buffered formalin, the brains were examined grossly and sectioned. Representative tissue sections from two lobes, basal ganglia, hippocampus, midbrain, pons, medulla and cerebellum were processed routinely in paraffin, and sections were stained with hematoxylin-eosin. Gross and microscopic examinations were performed by study neuropathologist (R.P.) to assess pathological findings in both brain parenchymal, meninges, and cerebral blood vessels. Autopsy findings were categorized into gross, and microscopic findings. Gross findings were visible without a microscopic and were subsequently confirmed microscopically. CI were further categorized according to pathologic findings as acute (tissue pallor with presence of necrotic neurons with eosinophilic cytoplasm and nuclear pyknosis), subacute (variable degrees of neutrophil and macrophage infiltration, capillary proliferation, and early astrocytic reaction at the lesion edge without cavitary changes), or remote (removal of necrotic tissue resulting in cavitary change and presence of residual reactive astrocytes from a gliotic scar at the periphery).16
Statistical Analysis
Continuous data were expressed as median ± interquartile range (IQR). Categorical data were expressed as number and percentage. Associations between continuous variables were compared using Mann–Whitney U test. Categorical variables were compared using Fisher’s exact test. p value less than 0.05 was considered significant. All analyses were conducted using R Statistical Software (R Core Team 2013. Vienna, Austria). Multivariable regression analysis was not performed due to a small sample size.
Results
Twenty-six LVAD patients were identified to have undergone postmortem neuropathologic evaluation and 21 patients met inclusion criteria (67% male, 62% white) with median age of 57 years (IQR=36–67) with the median support time of 51 days (IQR=15–393). The median time from death to autopsy was 19 hours (IQR=13–22). The most common indication for LVAD was dilated non-ischemic cardiomyopathy (48%) and the majority of patients met criteria for New York Heart Association III or IV prior to LVAD implantation (Table 1). All except 3 patients were implanted with a continuous-flow device and the most common device was HeartMate II (67%). Only 2 patients in this cohort had baseline MRI brain at 3 and 5 years prior to LVAD implantation, neither showed any evidence of small vessel disease or CMBs.
Table 1.
Patient Characteristics and complications during LVAD course
| Patient characteristics | All patients (n=21) |
|---|---|
| Age, years, median (IQR) | 57 (36–67) |
| Male, n (%) | 14 (67%) |
| Body mass index, median (IQR) | 29 (23–33) |
| Ethnicity, n (%) | 13 (62%) |
| White | 13 (62%) |
| African American | 7 (33%) |
| Hispanic | 1 (5%) |
| Pre-implant medical comorbidities, n (%) | |
| Diabetes mellitus | 8 (38%) |
| Hypertension | 11 (52%) |
| Arrhythmia | 13 (62%) |
| Chronic kidney disease | 6 (29%) |
| Pre-LVAD ischemic stroke | 1 (5%) |
| Pre-LVAD intracranial hemorrhage | 0 (0%) |
| Reason for LVAD implantation, n (%) | |
| Ischemic cardiomyopathy | 9 (42%) |
| Dilated non-ischemic cardiomyopathy | 10 (48%) |
| Congenital heart defect | 2 (10%) |
| Pre-LVAD implantation surgery NYHA class, median (IQR) | 3 (2–4) |
| LVAD support time, days, median (IQR) | 51 (15–393) |
| LVAD brand, n (%) | |
| Heartmate II | 14 (67%) |
| Heartmate III | 3 (13%) |
| HeartWare | 1 (5%) |
| Berlin Heart | 2 (10%) |
| Heartmate XVE | 1 (5%) |
| Known complications during LVAD support, n (%) | |
| Infection | |
| Bloodstream infection | 11 (52%) |
| Pump pocket | 1 (5%) |
| Driveline | 2 (10%) |
| Pump | 2 (10%) |
| 2 or more types of infection | 5 (24%) |
| Thrombotic/Bleeding complications | |
| Pump thrombosis | 2 (10%) |
| Deep vein thrombosis | 3 (14%) |
| Pulmonary embolism | 1 (5%) |
| Significant non-intracranial bleeding | 7 (43%) |
| Disseminated intravascular coagulation | 1 (5%) |
| Clinical cerebral injuries | 7 (33%) |
| Ischemic stroke | 3 (14%) |
| Hemorrhagic stroke | 2 (10%) |
| Global cerebral ischemia | 2 (10%) |
| More than one types of clinical cerebral injuries | 3 (14%) |
| Cause of Death, n (%) | |
| Multisystem organ failure | 8 (38%) |
| Sepsis | 4 (19%) |
| Devastating cerebral injuries | 5 (24%) |
| Transplant rejection | 1 (5%) |
| Non-intracranial hemorrhage | 2 (10%) |
| Right heart failure | 1 (5%) |
| Expired during LVAD implantation hospital stay, n (%) | 12 (57%) |
| Autopsy interval from death, hours, median (IQR) | 19 hours (13–22) |
IQR- interquartile range; LVAD, left ventricular assist device; NYHA, New York Heart Association.
During LVAD support, 11 (52%) patients had at least one bloodstream infection, of which 5 patients also had confirmed LVAD infections (2 pump infection, 1 pump pocket infection, 2 driveline infection). Ten (48%) patients had non-cerebral thrombotic (n=4) or bleeding complications (n=7) during LVAD support, of which 1 patient had both complications (Deep Venous Thrombosis [DVT], Disseminated Intravascular Coagulopathy and non-intracranial bleeding), and 2 patients had more than one types of thrombotic event (DVT and pump thrombosis) (Supplemental Table 1). Seven had clinically diagnosed cerebral injuries before death including 3 IS, 2 ICHs, and 2 global cerebral ischemia, of those 3 patients had more than one types of injuries. Twelve patients (57%) expired during the index hospitalization of LVAD implantation. The most common causes of death were multisystem organ failure (38%), followed by devastating cerebral injuries (24%) and sepsis (19%).
Neuropathologic Findings
Upon neuropathological examination, cerebral injury was present in all 21 patients (100%), and cerebral microvascular injury was present in 19 patients (90%). Autopsy findings (Table 2) were categorized as gross findings of CI (8, 38%), IPH (6, 29%), SAH (3, 14%), SDH (1, 5%) and microscopic findings of diffuse HIBI (2, 10%), microscopic parenchymal hemorrhage (12, 57%), perivascular hemorrhage (3, 19%), perivascular hemosiderin deposition (9, 42%), meningeal hemorrhage (5, 24%), and vascular changes (19, 90%) including arterial intimal fibrosis (15, 71%), vascular sclerosis (3, 14%) and perivascular inflammation (13, 62%). Fourteen (78%) patients had more than one types of cerebral injuries. However, only 7 (33%) patients were diagnosed ante-mortem, a clinically silent cerebral injury proportion of 67%. Detailed descriptions of autopsy findings of each patient are listed in Supplemental Table 1.
Table 2.
Brain Autopsy Findings in 21 Left Ventricular Assist Device Patients
| Brain autopsy finding | n (%) |
|---|---|
| Gross findings | |
| Cerebral infarction | 8 (38%) |
| Intraparenchymal hemorrhage | 6 (31%) |
| Subdural hematoma | 1 (4%) |
| Subarachnoid hemorrhage | 3 (14%) |
| Microscopic findings | |
| Cerebral microbleeds | 17 (81%) |
| Microscopic parenchymal hemorrhage | 12 (57%) |
| Perivascular hemorrhage | 3 (14%) |
| Perivascular hemosiderin deposition | 9 (42%) |
| Vascular changes | 19 (90%) |
| Arterial intimal fibrosis | 15 (71%) |
| Perivascular inflammation | 13 (62%) |
| Vascular sclerosis | 3 (14%) |
| Meningeal hemorrhage | 5 (24%) |
| Diffuse hypoxic ischemic brain injury | 2 (10%) |
| Microabscess | 1 (5%) |
Gross Findings
On gross examination, there were 8 patients with CI (38%) and 6 patients with ICH (29%), among which 5 patients had both. All ICHs were acute. All six ICH patients had IPH (Figure 1A), among which 3 patients also had SAH and 1 had SDH. Of 8 patients with CI, only 3 had clinical symptoms with a clinical silent CI proportion of 63%. Majority of patients (75%) had more than one infarct. Two patients had acute/subacute infarcts (Figure 1B), 2 had remote infarcts (Figure 1C), and 4 had both types. The most common locations of CI were lobar and hippocampus (6, 75%), followed by brainstem/cerebellum (3, 38%) and deep nuclei (1, 13%) (Supplemental Table 1). Half of IPH were multifocal in locations, and the most common location involved was lobar (4, 67%). Only two patients were diagnosed with IPH prior to death, a clinical silent proportion of 75%. Among the IPH patients, 4 patients (67%) also had evidence of CMBs on microscopic evaluation.
Figure 1.
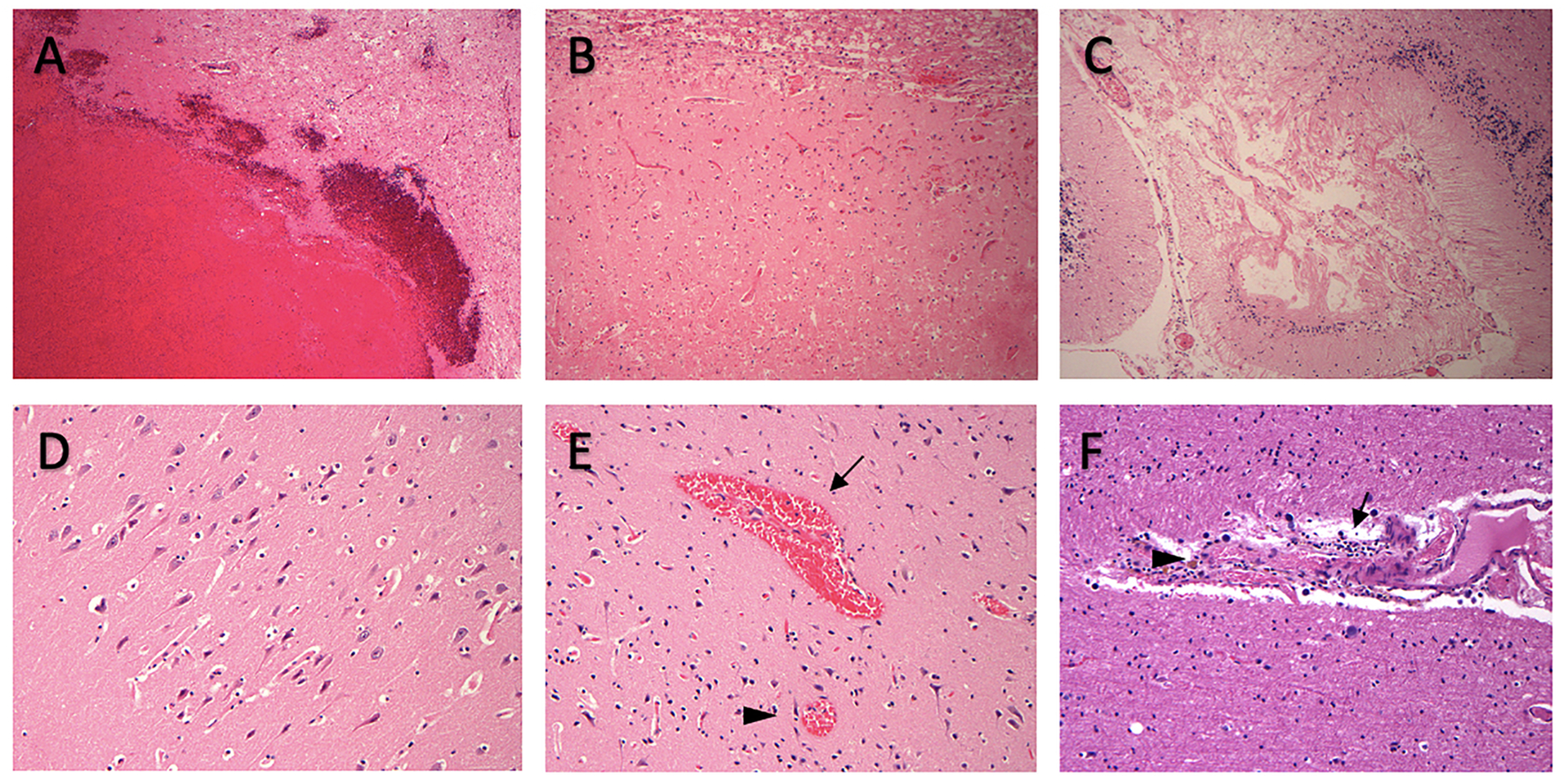
Representative neuropathological findings of cerebral injuries and vascular changes. On autopsy, 6 patients had evidence of acute intraparenchymal hemorrhage (A), 8 patients showed evidence of acute cerebral infarct (B) or chronic infarct (C). Two patients had evidence of diffuse hypoxic ischemic brain injury (D). Seventeen patients had cerebral microbleeds which includes microscopic parenchymal hemorrhage (E, arrowhead), perivascular hemorrhage (E, arrow) and perivascular hemosiderin deposition (F, arrowhead). Thirteen patients had perivascular inflammation (F, arrow).
Patients who developed thromboembolism events (deep vein thrombosis, pulmonary embolism and pump thrombosis) or infection during LVAD support were not found to be more likely to suffer from CI, or ICH. Both patients with CI and ICH had longer LVAD length of support time compared to patients without CI (372 days, IQR 118–762 vs. 49 days, IQR 7–244, p=0.04) or ICH (239 days, IQR=35–477 vs. 98 days, IQR=9–173, p=0.03).
Microscopic Findings
Of 21 patients, 5 patients (24%) had microscopic meningeal hemorrhage, 2 patients (10%) had diffuse HIBI (Figure 1D), and 17 (81%) patients had CMBs. CMBs included microscopic intraparenchymal hemorrhage (n=12) (Figure 1E), perivascular hemorrhage (n=3) (Figure 1E), and perivascular hemosiderin deposition (n=9) (Figure 1F). Majority of patients (76%) had multi-focal CMBs, the locations involved were brainstem (n=12), lobar (n=9), deep nuclei (n=9), hippocampus (n=7), and cerebellum (n=4).
Eight patients (47%) had at least one bacteremia or LVAD infection during LVAD course, but no association was found between bacteremia or LVAD infection and presence of CMBs on autopsy (p=0.59). Among patients with CMBs, 3 patients (18%) also had acute IPH and 5 patients (29%) had CI. Presence of CMBs was not associated with the presence of IPH (p=0.051) or CI (p=0.2). In addition, diabetes mellitus, hypertension, cardiac arrhythmia, chronic kidney disease, non-cerebral thromboembolism or bleeding events, length of LVAD support, were not found to be associated with CMBs (Table 3).
Table 3.
Comparison of Patients with and without Cerebral Microbleeds
| Patient characteristics | Patient with CMBs n=17 |
Patient without CMBs n=4 |
P value |
|---|---|---|---|
| Age, median (IQR) | 48 (31–63) | 64 (58–69) | 0.12 |
| Male, n | 13 (76%) | 1 (25%) | 0.28 |
| Body mass index, median (IQR) | 29 (26–33) | 24 (23–28) | 0.34 |
| Past Medical History, n | |||
| Diabetes mellitus | 7 (41%) | 1 (25%) | 1 |
| Hypertension | 9 (53%) | 2 (50%) | 1 |
| Arrhythmia | 11 (65%) | 2 (50%) | 0.6 |
| Chronic kidney disease | 5 (29%) | 1 (25%) | 1 |
| Pre-LVAD ischemic stroke | 0 | 1 (25%) | 0.22 |
| Pre-LVAD hemorrhagic stroke | 0 | 0 | NA |
| LVAD support time, days, median (IQR) | 46 (7–239) | 612 (337–853) | 0.02 |
| LVAD brand- Heartmate II | 11 (65%) | 3 (75%) | 1 |
| Complications during LVAD course, n (%) | |||
| Bloodstream or LVAD Infections | 9 (53%) | 3 (75%) | 0.59 |
| Cerebral infarct | 5 (29%) | 3 (75%) | 0.25 |
| Intraparenchymal hemorrhage | 3 (17%) | 3 (75%) | 0.06 |
| Pump thrombosis | 1 (6%) | 1 (25%) | 0.35 |
| Deep vein thrombosis | 3 (17%) | 0 | 1 |
| Pulmonary embolism | 0 | 1 (25%) | 1 |
| Significant non-intracranial bleeding | 7 (41%) | 2 (50%) | 0.58 |
| Disseminated intravascular coagulation | 1 (6%) | 0 | 1 |
IQR- interquartile range; LVAD, left ventricular assist device
Nineteen patients (90%) had vascular changes noted on microscopic examination, of which 15 patients had arterial intimal fibrosis, 13 patients had perivascular inflammation (Figure 1F), and 3 patients had vascular sclerosis. Among the patients with perivascular inflammation, majority (54%) affected blood vessels in multiple locations and the common locations involved were lobar (n=7), deep nuclei (n=6) and brainstem (n=5). A majority of the patients with perivascular inflammation also had evidence of CMBs (85%), 2 patients had IPH and 4 patients had CI. Although no direction association was found between presence of perivascular inflammation and incidence of IPH (p=0.1), CI (p=0.7) or CMBs (p=0.6). Of those 13 patients, 7 patients (54%) had at least one bacteremia and/or device infection during their LVAD course, 2 patients (15%) had active infection before autopsy and 1 patient had evidence of microabscess on autopsy. But there was no association between bacteremia and presence of perivascular inflammation on autopsy (p=1) in this cohort.
Nine patients died within 4 weeks post LVAD implantation surgery and during the same index of hospital admission due to surgical or post-operative related complications including 5 patients from persistent multisystem organ failure, 3 patients from post-operative persistent bleeding and hemorrhagic shock, 1 patient from global cerebral ischemia from hypotension during surgery. Twelve patients were on long term LVAD support. During subanalysis of these 12 patients (median length of support 340 days, IQR 151–627), 6 patients had cerebral infarction, 6 patients had ICH, 6 patients had CMBs and 6 patients had evidence of perivascular inflammation. There is no significant difference found in the prevalence of CI, CMBs, and perivascular inflammation, but ICH is more prevalence in patients with long term LVAD support (p=0.01) (Supplemental table 2).
Discussion
Our study of brain pathology among those with LVAD support showed high prevalence of CMBs (81%) and cerebral microvascular injuries (90%). The high prevalence of CMBs seen in our LVAD cohort is similar to prior studies on MRI after LVAD explantation (~97–98%),13,17 and is much higher than the reported prevalence of 5–6 % in the general elderly population.18 The presence of CMBs are associated with increased risk for symptomatic hemorrhagic stroke and cognitive decline14, 19, and now is considered a biomarker for cerebral microvascular injuries.20,21
The pathophysiology of CMBs currently unknown in LVAD patients, and our study provides an insight on ongoing cerebral microvascular injury as a mechanism of CMBs. LVAD patients experience ongoing and chronic inflammation due to frequent infections and foreign body reaction. Perivascular inflammation was commonly observed indicating ongoing vascular inflammation in LVAD patients, similar to infectious endocarditis.22 Autopsy findings of intracranial perivascular inflammation without an obvious mycotic aneurysm was seen in patients with infective endocarditis who suffered from ICH.23 Chronic vascular inflammation is associated with breakdown of the blood brain barrier and increased vascular permeability,24 which may play a role in developing CMBs and cerebral microvascular injuries seen with LVAD. A study observed a possible trend between the number of CMBs and LVAD-related infection.14 However, we did not observe this association in our study. In addition to chronic inflammation, CF-LVAD patients also frequently suffered from non-intracranial bleeding due to acquired von Willebrand factor dysfunction, a marker for endothelial dysfunction.25,26 The lack of physiologic cardiac cycle in CF-LVAD resulting in impaired endothelial cell function and autoregulation may play a role in the high prevalence of cerebral microvascular injuries.27 Although our study included pulsatile-flow LVAD, due to the small sample size, no significant differences in rate of cerebral microvascular injuries were observed between different types of LVAD.
Furthermore, the length of LVAD support was not found to be associated with higher risk for CMBs. High prevalence of CMBs in LVAD may also be related to recent cardiac surgery and cardiopulmonary bypass, as study has found 76% of patients had new CMBs post- cardiac surgery with cardiopulmonary bypass.28 In addition, during LVAD support, patients are on strict and aggressive anti-thrombotic therapy to prevent thrombosis. These specific circumstances related to LVAD support may result in cerebral microvascular damages and high prevalence of CMBs in this population. While these findings provide clues to mechanisms of cerebral vascular injury in LVAD population, further studies of experimental neuropathology are needed to determine causal relationships.
Our study revealed a high prevalence of silent infarcts (67%), which is not surprising given the high prevalence of clinical strokes in the cohort. This finding is consistent with another published autopsy study of 24 patients with LVAD.29 Only 1 in ~10 infarcts detected on MRI are reported to be symptomatic in cohort studies.30,31 Implementation of neuromonitoring protocols and directed anticoagulation management, designed to detect and prevent stroke in LVAD patients, may significantly improve silent brain injury rates in these patients.32
Our study has several limitations. The small convenient sample size of autopsy patients does not represent all patients on LVAD support, most of whom survive. Our study is also retrospective, and the brain autopsy was not conducted with the research hypothesis of cerebral vascular injury. But the archived slides were reviewed by a neuropathologist for this study. While there was standard sampling protocol of brain section for microscopic review, there is sampling bias since certain brain regions, including hippocampus, were always sampled. Relative to the brain volume, cerebral cortex was under-sampled. CMBs and other findings are underestimated in our study, since brain regions are selected for microscopic evaluations. In the absence of a control group without LVAD, and lack of baseline MRI brain prior to LVAD implantation, it is difficult to conclude whether these findings in our study were associated with LVAD support or with heart failure and its comorbidities and complications.
Conclusions
Our post-mortem analysis demonstrated a high prevalence of cerebral microvascular injuries and CMBs among LVAD patient. Although the cause of frequent cerebral injuries this population is multi-factorials, our finding provides some insight to the underlying mechanism. Further research is warranted to better understand the underlying pathogenesis of LVAD-associated brain injuries.
Supplementary Material
Footnotes
Conflicts of interest/Disclosure:
Dr. Ken Uchino serves on the data safety monitoring board of a study of LVAD device sponsored by Evaheart, Inc., and has served on the clinical endpoint adjudication committee for a clinical trial by Abbott Laboratories, unrelated to heart failure or LVADs.
Dr. Tracey H. Fan has has nothing to disclose.
Dr. Sung-Min Cho has nothing to disclose.
Dr. Catherine E. Hassett has no disclosure.
Dr. Randall C. Starling has nothing to disclose.
Dr. Richard Prayson has nothing to disclose.
Ethic approval: This study was approved by the local institutional review board.
Consent for publication: All authors have read and give consent for publication.
Availability of data and material:
The data that support the findings of this study are available on request from the corresponding author
Reference
- 1.Cho SM, Moazami N, Frontera JA. Stroke and Intracranial Hemorrhage in HeartMate II and HeartWare Left Ventricular Assist Devices: A Systematic Review. Neurocrit Care. 2017;27(1):17–25. [DOI] [PubMed] [Google Scholar]
- 2.Acharya D, Loyaga-Rendon R, Morgan CJ, et al. INTERMACS Analysis of Stroke During Support With Continuous-Flow Left Ventricular Assist Devices: Risk Factors and Outcomes. JACC Hear Fail. 2017;5(10):703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho S-M, Hassett C, Rice CJ, Starling R, Katzan I, Uchino K. What Causes LVAD-Associated Ischemic Stroke? Surgery, Pump Thrombosis, Antithrombotics, and Infection. ASAIO J. 2019;65(8):775–780. [DOI] [PubMed] [Google Scholar]
- 4.Starling RC, Moazami N, Silvestry SC, et al. Unexpected Abrupt Increase in Left Ventricular Assist Device Thrombosis. N Engl J Med. 2014;370(1):33–40. [DOI] [PubMed] [Google Scholar]
- 5.Trachtenberg BH, Cordero-Reyes AM, Aldeiri M, et al. Persistent blood stream infection in patients supported with a continuous-flow left ventricular assist device is associated with an increased risk of cerebrovascular accidents. J Card Fail. 2015;21(2):119–125. [DOI] [PubMed] [Google Scholar]
- 6.Yoshioka D, Sakaniwa R, Toda K, et al. Relationship between bacteremia and hemorrhagic stroke in patients with continuous-flow left ventricular assist device. Circ J. 2018;82(2):448–456. [DOI] [PubMed] [Google Scholar]
- 7.Lee T, Buletko AB, Matthew J, Cho SM. Bloodstream infection is associated with subarachnoid hemorrhage and infectious intracranial aneurysm in left ventricular assist device. Perfus (United Kingdom). 2020;35(2):117–120. [DOI] [PubMed] [Google Scholar]
- 8.Chiang YP, Cox D, Schroder JN, et al. Stroke risk following implantation of current generation centrifugal flow left ventricular assist devices. J Card Surg. 2020;35(2):383–389. [DOI] [PubMed] [Google Scholar]
- 9.Mehra MR, Goldstein DJ, Uriel N, et al. Two-Year Outcomes with a Magnetically Levitated Cardiac Pump in Heart Failure. N Engl J Med. 2018;378(15):1386–1395. [DOI] [PubMed] [Google Scholar]
- 10.Lateef N, Usman MS, Colombo PC, et al. Meta-Analysis Comparing Risk for Adverse Outcomes After Left Ventricular Assist Device Implantation in Patients With Versus Without Diabetes Mellitus. Am J Cardiol. 2019;124(12):1918–1923. [DOI] [PubMed] [Google Scholar]
- 11.Tsiouris A, Heliopoulos I, Mikroulis D, Mitsias PD. Factors defining occurrence of ischemic and hemorrhagic strokes during continuous flow left ventricular assist device support. Gen Thorac Cardiovasc Surg. 2020;68(4):319–327. [DOI] [PubMed] [Google Scholar]
- 12.Murase S, Okazaki S, Yoshioka D, et al. Abnormalities of brain imaging in patients after left ventricular assist device support following explantation. J Hear Lung Transplant. 2020;39(3):220–227. [DOI] [PubMed] [Google Scholar]
- 13.Yoshioka D, Okazaki S, Toda K, et al. Prevalence of cerebral microbleeds in patients with continuous-flow left ventricular assist devices. J Am Heart Assoc. 2017;6(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bokura H, Saika R, Yamaguchi T, et al. Microbleeds are associated with subsequent hemorrhagic and ischemic stroke in healthy elderly individuals. Stroke. 2011;42(7):1867–1871. [DOI] [PubMed] [Google Scholar]
- 15.Kirklin JK, Naftel DC, Pagani FD, et al. Pump thrombosis in the Thoratec HeartMate II device: An update analysis of the INTERMACS Registry. J Hear Lung Transplant. 2015;34(12):1515–1526. [DOI] [PubMed] [Google Scholar]
- 16.Yachnis AT. Chapter 2 Vascular Disease. Neuropathology: Foundations in Diagnostic Pathology. Second Edi. (Prayson R, ed.). Elsevier Inc.; 2017. [Google Scholar]
- 17.Murase S, Okazaki S, Yoshioka D, et al. Abnormalities of brain imaging in patients after left ventricular assist device support following explantation. J Heart Lung Transplant. November 2019. [DOI] [PubMed] [Google Scholar]
- 18.Janaway BM, Simpson JE, Hoggard N, et al. Brain haemosiderin in older people: Pathological evidence for an ischaemic origin of magnetic resonance imaging (MRI) microbleeds. Neuropathol Appl Neurobiol. 2014;40(3):258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akoudad S, Wolters FJ, Viswanathan A, et al. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol. 2016;73(8):934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8(2):165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Soo YOY, Mok VCT. Cerebral microbleeds: Is antithrombotic therapy safe to administer? Stroke. 2014;45(9):2811–2817. [DOI] [PubMed] [Google Scholar]
- 22.Karstrup CC, Jensen HE, Aalbæk B, Leifsson PS, Boye M, Agerholm JS. Endocarditis-associated Brain Lesions in Slaughter Pigs. J Comp Pathol. 2011;144(4):289–295. [DOI] [PubMed] [Google Scholar]
- 23.Masuda J, Yutani C, Waki R, Ogata J, Kuriyama Y, Yamaguchi T. Histopathological analysis of the mechanisms of intracranial hemorrhage complicating infective endocarditis. Stroke. 1992;23(6):843–850. [DOI] [PubMed] [Google Scholar]
- 24.Pober JS, Sessa WC. Inflammation and the blood microvascular system. Cold Spring Harb Perspect Biol. 2015;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crow S, Chen D, Milano C, et al. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann Thorac Surg. 2010;90(4):1263–1269. [DOI] [PubMed] [Google Scholar]
- 26.Lip GYH, Blann A. von Willebrand factor: A marker of endothelial dysfunction in vascular disorders? Cardiovasc Res. 1997;34(2):255–265. [DOI] [PubMed] [Google Scholar]
- 27.Witman MAH, Garten RS, Gifford JR, et al. Further Peripheral Vascular Dysfunction in Heart Failure Patients With a Continuous-Flow Left Ventricular Assist Device: The Role of Pulsatility. JACC Hear Fail. 2015;3(9):703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel N, Banahan C, Janus J, et al. Perioperative Cerebral Microbleeds after Adult Cardiac Surgery. Stroke. 2019;50(2):336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kannapadi NV, White B, Choi CW, Chen LL, Cho S-M. Clinically Silent Brain Injury and Perioperative Neurological Events in Patients with Left Ventricular Assist Device: A Brain Autopsy Study. ASAIO J. 2020;Available. [DOI] [PubMed] [Google Scholar]
- 30.Longstreth WT, Dulberg C, Manolio TA, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: The Cardiovascular Health Study. Stroke. 2002;33(10):2376–2382. [DOI] [PubMed] [Google Scholar]
- 31.Cho SM, Deshpande A, Pasupuleti V, Hernandez AV., Uchino K. Radiographic and clinical brain infarcts in cardiac and diagnostic procedures a systematic review and meta-analysis. Stroke. 2017;48(10):2753–2759. [DOI] [PubMed] [Google Scholar]
- 32.Fan Tracey H.; Hassett Catherine E.; Migdady Ibrahim; Price Carrie; Choi Chun Woo; Katzan Irene; Cho S-M. How Are We Monitoring Brain Injuries in Patients With Left Ventricular Assist Device? A Systematic Review of Literature. ASAIO J. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author


