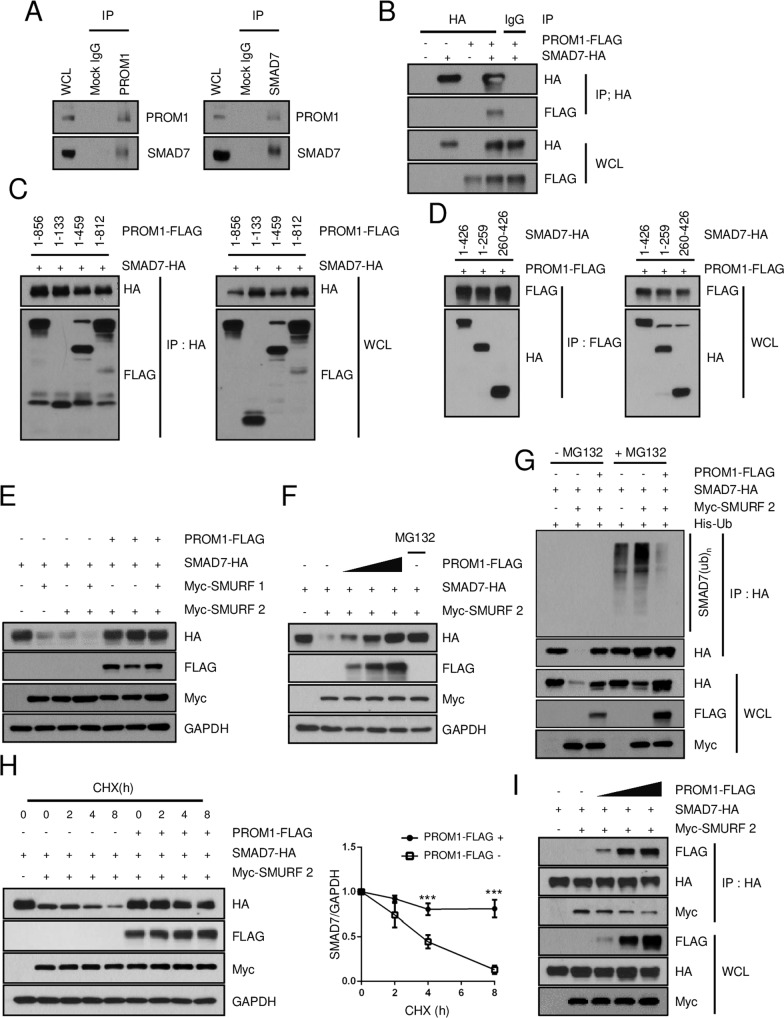
Fig. 6. PROM1 stabilizes the SMAD7 protein.
A The molecular association of PROM1 with SMAD7 was determined by reciprocal endogenous immunoprecipitation (IP) in the livers of 9-week-old male mice that were subjected to BDL for 1 week. B The molecular interaction between PROM1 and SMAD7 was determined by IP after PROM1-FLAG and SMAD7-HA were transfected into HEK293T cells. C, D Domain analysis was performed by IP after different truncated mutants of PROM1-FLAG and SMAD7-HA with the indicated combinations were used to transfect HEK293T cells. E After the transient expression of SMAD7-HA, Myc-SMURF-1, Myc-SMURF-2, and PROM1-FLAG at the indicated combinations in HEK293T cells, the expression level of each protein was determined by immunoblotting. F Different amounts of PROM1-FLAG (0, 2, and 4 μg) were transiently expressed, along with SMAD7-HA and Myc-SMURF2, in HEK293T cells. The expression level of each protein was determined by immunoblotting. MG132 (2.5 µM) treatment was used as a positive control for SMAD7-HA. G SMAD7 ubiquitination was determined by IP after the cotransfection of PROM1-FLAG, SMAD7-HA, Myc-SMURF2, and His-ubiquitin (His-Ub) in the presence of MG132 (2.5 µM). H HEK293T cells were cotransfected with PROM1-FLAG, SMAD7-HA, and Myc-SMURF2 at the indicated combinations and treated with 2 μg/ml cycloheximide (CHX) for the indicated times. SMAD7 protein levels were determined by immunoblotting (left panel). The relative protein expression of SMAD7, shown in the left panel, was statistically analyzed from three independent experiments (right panel). I The molecular interaction of SMAD7-HA with Myc-SMURF2 and PROM1-FLAG was assessed by co-IP after the transfection of SMAD7-HA, Myc-SMURF2, and different amounts of PROM1-FLAG (0, 2, and 4 μg). WCL, whole-cell lysate; IP, immunoprecipitation. ***p < 0.001. All data are the mean ± S.E.M.