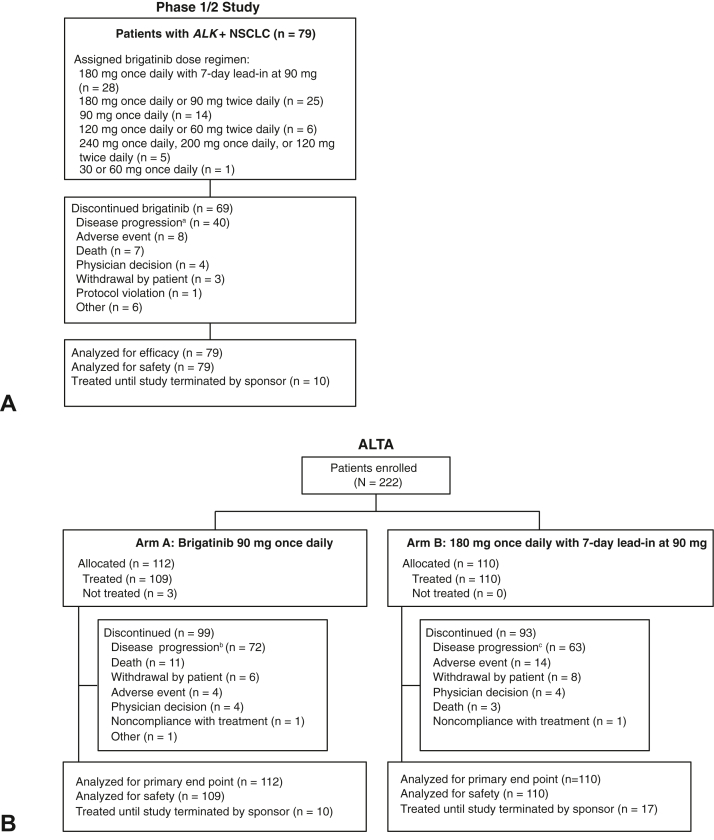
Figure 1.
CONSORT diagrams for (A) the phase 1/2 study and (B) the ALTA trial. aA total of 33 patients had documented disease progression per RECIST version 1.1. Seven patients had clinical disease progression; bA total of 63 patients had documented disease progression per RECIST version 1.1. Nine patients had clinical disease progression; cA total of 50 patients had documented disease progression per RECIST version 1.1. A total of 13 patients had clinical disease progression. ALK+, ALK rearrangement positive; RECIST, Response Evaluation Criteria in Solid Tumors.