Abstract
Alcoholic neuropathy (AN), a debilitating condition that mainly affects chronic alcohol drinkers, is thought to cause lesions in the peripheral nervous system leading to sensory, autonomic, and motor dysfunctions. Despite many studies, the pathogenesis of these lesions is still not completely understood. We investigated few aspects on the development of alcohol-induced peripheral neuropathy, by assessing sensory, motor and autonomic functions, as well as stereological analysis of axonal fibers and myelin sheath of the sciatic nerve. Twelve male Wistar rats were divided into Control group and Alcohol group that was submitted to Two Bottle-Choice Paradigm of intermittent and voluntary alcohol solution intake (20%; v/v) during eight weeks. At the end of treatment, three different sensorium-motor tests were applied - Tactile Sensitivity, Thermal Sensitivity, and Functional Observational Battery (FOB). Quantitative morphometric analysis of sciatic nerve structures was performed by stereological method. Alcohol concentration in the blood was measured to analyze possible correlation between availability of alcohol in the blood and the magnitude of the peripheral nerve lesion. Our data showed a peripheral effect of chronic alcohol intake associated with hyperalgesia and a process of demyelination with a strong correlation with alcohol consumption. This process was associated with increased tactile sensitivity, with behavioral reflexes such as locomotor hyperactivity, changes in gait and balance, and autonomic reflexes such as piloerection.
Keywords: Alcoholic neuropathy, Myelin sheath, Functional Observational Battery (FOB), Stereology, Motor alterations, Sensitive alterations
Highlights
-
•
Alcoholic neuropathy and its consequences.
-
•
Neuropathy and sensitive and motor alterations.
-
•
Alcoholic neuropathy and thinner myelin sheath thickness.
Introduction
Alcohol Use Disorder (AUD) is a chronic and progressive condition involving young people and adults worldwide (Diagnostic and Statistical Manual of Mental Disorders-5; World Health Organization, 2018). A recent global alcohol abuse report indicated that approximately 3 billion people consume alcohol worldwide (Global Status Report on Alcohol and Health, 2016). Alcohol is one of the most consumed psychotropic substances worldwide, being classified as the seventh major risk factor for death and responsible for more than 200 pathological conditions, such as Peripheral or Alcoholic Neuropathy (AN) (Burton and Sheron, 2018, Griswold et al., 2018, World Health Organization, 2018).
AN is a progressive and extremely debilitating complication of chronic alcohol consumption (with an incidence of 66%), which can result in sensory, motor and autonomic dysfunctions, as well as axonal degeneration of the peripheral nervous system (Mellion et al., 2011; Kanwaljit Chopra and Tiwari, 2012; Nguyen et al., 2012; Donnadieu-Rigole et al., 2014; Maiya and Messing, 2014). AN symptoms are known as neuropathic pain (high-intensity chronic discomfort, with a low response to available analgesic drugs) (Duehmke et al., 2017); allodynia (pain caused by a stimulus that is usually not painful); hyperalgesia (increased pain sensation from a painful stimulus); and paresthesia (burning or prickling sensation without motor stimulus) (Kanwaljit Chopra and Tiwari, 2012; Kaur et al., 2017).
Despite being considered as axonal-predominant neuropathy, Koike et al. (2001), important abnormalities in the myelin sheath after chronic alcohol consumption are observed, such as irregularities and demyelination, with enlargement of the nodes of Ranvier (Koike et al., 2001).
Several studies on nerve conduction in individuals with AN showed a decrease on the amplitude of sensory potentials in the extremities and mean conduction deceleration. In these cases, motor conduction velocity may also be reduced, which could be associated with increased motor latency (Koike et al., 2001, Maiya and Messing, 2014, Zambelis et al., 2005). Electromyographic studies, such as Behse & Buchthal (1977), observed in a sample of chronic drinkers, signs of denervation (decreased recruitment pattern, fibrillation, and marked positive wave) with significant weakness in lower limbs (Behse and Buchthal, 1977).
The etiology of AN has been investigated for decades and it is thought to be a multifactorial process, mediated mainly by the toxic effect of alcohol consumption, which can also be aggravated by other factors, such as thiamine deficiency and malnutrition (Koike et al., 2001, Mellion et al., 2014). Recent studies related to the mechanisms by which the toxic effect of alcohol can trigger AN resulted in several hypotheses such as activation of spinal cord microglia, activation of metabotropic glutamate receptor 5 (MGLU5), oxidative stress, the release of proinflammatory cytokines, activation of protein kinase C, extracellular signaling kinases, high concentrations of acetaldehyde, and activation of hypothalamic-pituitary-adrenal axis (HPA) (Kandhare et al., 2012; Kanwaljit Chopra and Tiwari, 2012; Fu et al., 2015).
One of the first symptoms of AN is a slowly progressive sensory-dominant neuropathy, which affects motor and autonomic functions, being related to the amount and duration of alcohol consumption (Chopra and Twari, 2012).
Although many studies have been done, so far there is not reliable treatment for AN due to the lack of understanding of its pathophysiology. Nevertheless, the use of some functional tests, such as the tactile sensitivity test, thermal sensitivity test, and the Functional Observation Battery (FOB) can shed some light with the attempt to give a more detailed functional view of the peripheral lesion evolution, comprising more than 30 parameters in the autonomic, neuromuscular, sensorimotor, and behavioral domains (Redferm et al., 2005). Furthermore, as axonal injury and demyelination of sensory and motor fibers are considered the biological basis of peripheral nervous system alterations in individuals who ingest significant amounts of alcohol (Chopra and Twari, 2012), morphometric analysis of these structures allows the assessment of the degree of nerve damage. Stereology is a technique that allows obtaining accurate information and unbiased estimates of the number and diameter of axons and myelin sheath from a small sample. Mayhew (1988) was the first to use the fractionation technique (Gundersen, 1986) to estimate the total number of myelinated axons in the tibial nerve of rats. It is a reliable method to assess alcohol induced tissue damage (Gundersen, 1986). These analyzes can contribute to a better understanding of the AN pathogenesis (Chopra and Twari, 2012).
Therefore, the present study aimed to investigate the development of AN during chronic alcohol intake, by assessing sensory (tactile and thermal sensibility tests), motor and autonomic functions (Functional Observational Battery; FOB), as well as stereological analysis of axonal fibers and myelin sheath of the sciatic nerve.
Experimental procedures
Animals
Twelve male Wistar rats (60 days old), from Centro de Desenvolvimento de Modelos Experimentais para Biologia e Medicina (CEDEME) of Universidade Federal de São Paulo (UNIFESP), were kept under controlled environmental conditions (21 ± 1 °C, 12 h light / dark cycle and access to water and food ad libitum). For all animals, food intake and body weight were measured twice a week.
The animals were divided into two groups: Control group (CO; n = 6) and Alcohol group (AL; n = 6). The CO group did not consume alcohol, while the AL group consumed alcohol following the Two-Bottle Choice Paradigm protocol for eight weeks. To evaluate the chronic effects of the substance, immediately after the last alcohol exposure, both groups underwent Functional Observational Battery (FOB) and collection of the sciatic nerve for stereological analysis after perfusion. Thus, these procedures were performed when the animals were under the effect of chronic alcohol intake. The Tactile and Thermal sensitivity tests were performed one day before to avoid interference with the FOB behavioral tests.
Experimental procedures were performed according to Colégio Brasileiro de Experimentaç ão Animal (COBEA) and approved by the Research Ethics Committee - UNIFESP (protocol n°. 8559100615). All efforts were made to minimize the number of animals used and their suffering (these animals were the same used in a previous study by our group - Conte et al., 2019a, Conte et al., 2019b – with stereological analysis of the brain).
Alcohol administration protocol
Alcohol administration protocols that induce nervous tissue damage vary from a four-day acute intoxication (Crews et al., 2004) to 40 weeks of chronic consumption (Dlugos, 2006). In this study, we utilized a protocol with free access and choice between a bottle containing alcohol solution and another containing water. The free access to the alcohol bottle was on Mondays, Wednesdays, and Fridays. On the other days, the animals had access to two bottles of water. The position of the ethanol bottle was alternated in each drinking session to avoid the interference of conditioned place preference, according to Hwa et al. (2014). The ethanol solution (20% v.v.) was prepared with filtered water and 95% alcohol (Hwa et al., 2014). This protocol is a voluntary, self-administration model, mainly devoid of aversive stimulation. As there is no specific amount of alcohol known to induce peripheral neuropathy (Chopra and Twari, 2012) the voluntary intake protocol was an adequate choice to avoid stressful stimuli by treatment. Conversely, we assured that the animals would be exposed to eight weeks of treatment as it is a time length capable of inducing systemic changes to reproduce alcohol-related peripheral neuropathy (Mellion et al. 2013).
The residual volume of the alcohol solution and the water was measured daily in milliliters and grams (Hwa et al., 2014).
Tactile sensibility test
Both function and integrity of the afferent fibers can be inferred by the tactile response that is impaired in neuropathic conditions. To evaluate the tactile sensitivity of animals, the sensibility test with the Von Frey monofilaments (Touch Test™ Sensory Evaluator Kit of 20 - Leica Biosystems, Germany) was conducted, which is inexpensive and used in the clinical settings as well. These monofilaments were used in an increasing order of thickness (starting at 6 g), on the plantar surface of the pelvic limb of the animal only when it was immobile and standing on the four limbs on a mesh floor. The monofilaments were applied until they bent slightly and were held for two seconds. A limb withdrawal response was valid when the foot was completely removed from the platform (Pitcher et al., 1999). The monofilaments were applied five times at intervals of five seconds, or as soon as the pelvic limb was properly positioned on the platform. If the withdrawal response did not occur in five applications of a particular filament, then the next monofilament would be applied in ascending order of thickness.
Thermal Sensibility test
Likewise, thermal sensitivity alterations are common in neuropathic patients and easily evaluated in animal models. This test is a widely used and safe test that consists of a cold object (ice stick at −20 °C) applied to the center of the pelvic limbs (paw pads) of the animals five times, in a five-minute interval, to avoid desensitization. If there was no withdrawal response, the thermal stimulus was removed after 30 s. The latency of the last four trials was used to calculate the mean withdrawal latency from each animal in each pelvic limb. The thermal sensitivity was evaluated after the tactile sensitivity test (Miller et al., 2013).
Functional Observational Battery (FOB)
This test is used to detect chemical-induced sensorimotor impairments. It includes more than 30 independent observation parameters, which are grouped into domains. The domains are neurological, autonomic, and behavioral; each one with its measurement and evaluation parameters (Boucard et al., 2010). This test is commonly used in studies of neuropathic disorders, and it is easily replicable. FOB was performed immediately after last session of alcohol and before perfusion by two independent observers, who examined the reactivity of each animal to manipulation and stimuli, such as behavioral changes, motor activity, coordination, and sensory-motor reflex responses.
For the neurological domain, we evaluated the muscle tone parameters (forelimb grip strength and hypotonia), gait and equilibrium parameters (righting reflex and gait), and CNS excitation parameters (twitches, clonic and tonic convulsions). Regarding the autonomic domain, we evaluated lacrimation, pupil size, palpebral closure, salivation, piloerection, and breathing parameters. The behavioral domain was assessed by observation of spontaneous activity (hyperactivity), affective response (reactivity to catching and handling, defecation, and urination), and sensorial responses (touch response and tail-pinch response). On test day, the reactivity of each animal was examined by manipulation and stimuli while they were still in their cages or when placed in the arena of the open field test.
Firstly, the reactivity to catching the animal in its cage was analyzed, and the difficulty of removal was observed and classified as (normal: 0; little difficulty: 1; difficult: 2) depending on difficulty removing the animal from the cage (flight, aggression, vocalization); after that, we analyzed lacrimation (absence: 0, presence: 1); piloerection (absence: 0, moderate: 1, marked: 2); reactivity to handling (scored from 0 to 2 depending on difficulty encountered handling; e.g., aggression, vocalization), and pupil size (myosis: 1, mydriasis: 2).
Then, the animal was transferred to the open field arena where it was observed for 2 min regarding palpebral closure (drooping of the upper eyelids; normal: 0, moderate: 1, marked: 2); twitches (absence: 0, moderate: 1, marked: 2); clonic or tonic convulsions (absence: 0, presence: 1); locomotor hyperactivity (normal: 0, moderate: 1, high: 2); hypotonia (severe loss of strength: 0; moderate loss: 1; normal: 2; excessive resistance: 3); gait (normal: 0, abnormal: 1); breathing (normal: 0; increased: 1; to severely impaired - e.g., suffocation, hyperventilation: 2); and defecation/urination (scored from 0 to 2 depending on appearance—normal, colored, or soft feces—and quantity—excessive or normal). All the procedures were video recorded.
Soon after this initial stage of observation, the animal was evaluated in arena of the open field for reactivity to manipulations and stimuli, such as touch response, and tail pinch response. To evaluate the touch response, a gentle touch with a pen was performed on the side of the animal and observed its reaction (0: normal; 1: frightened; 2: aggressive). For tail-pinch response, we used curved tweezers applying a moderate pressure 2-cm above the extremity of the tail for ∼1 s (0: normal; 1: no reaction; 2: violent reaction).
The last evaluations were to determine the righting reflex, after holding the animal by the dorsal region and raising it 30 cm above the test platform (0: fall with all four paws, 1: all paws do not touch the ground, 2: fall laterally, 3: fall). Finally, we evaluated forelimb grip strength positioning the anterior limbs in the cage and performing a slight pull of the body (severe loss of strength: 0; moderate loss: 1; normal: 2; excessive resistance: 3) (Boucard et al., 2010).
To quantify and describe the effect of alcohol in each parameter of the neurological, autonomic, and behavioral domains, we used the “Mean Severity Score” (MSS). To calculate MSS per group is utilized the sum of the “mean scores” evaluated for each parameter that is included in a particular domain using the following equation:
| MSS = ∑ (i·ni) / n |
Where: i represents the number of grades attributed to a parameter, ni represents the number of animals reaching the score i, and n represents the group size.
Thus, MSS can be interpreted as: no effect of substance (MSS smaller than or equal to 2); moderate effect of substance (MSS between 3 and 4), and great effect of substance (MSS greater than 4) (Boucard et al., 2010).
Perfusion and Nerve Dissection
At the end of the FOB test, the animals were anesthetized by intraperitoneal injection with a mixture of 1 ml/kg of a ketamine (40 mg/kg), xylazine (20 mg/kg), fentanyl (0.3–0.5 mg/kg), and acepromazine (1 ml/kg).
The perfusion procedure was performed transcardially with saline 0.9% (100 ml) followed by a fixative solution containing 4% paraformaldehyde (500–700 ml) in an aqueous solution from paraformaldehyde heated to 60–65 °C (pH 9.5 at 4 °C) for 25 min (Conte et al., 2019a, Conte et al., 2019b, Wscieklica et al., 2019).
The dissection of the sciatic nerves was performed at the origin of the nerve between L5 and S1 segments to tibiofibular bifurcation. The measurement of this segment was carried out with a pachymeter. The nerve was sectioned into five equidistant slices. Each slice was fixed in buffered 2.5% glutaraldehyde and stored in the same fixative for subsequent dehydration and embedding in Epon resin 812. Semi-thin slices (500 nm), one cut for each slice totaling five cuts per nerve were performed with the aid of an ultramicrotome (Leica Inc UC6 - Wetzlar/Germany) and were subsequently stained with alcoholic toluidine blue and analyzed by the stereological method.
Alcohol Concentration Analysis
Right before the beginning of the perfusion, 0.5 ml of blood from the left ventricle was collected. The samples were placed into heparinized tubes and centrifuged at 2300 rpm, 4 ºC for 15 min (Biochain, Newark, CA, USA). Blood alcohol concentration analysis was performed by the method of spectrophotometry with the enzyme kit for the enzyme NAD-ADH (Conte et al., 2019a, Conte et al., 2019b, Wscieklica et al., 2019).
Stereological Analysis
Stereological analyses were performed in each nerve sampled in a systematic uniform random sampling (SURS) scheme (Raimondo et al., 2009).
Total number of myelinated axons from sciatic nerve (Naxons)
The total number of axons was estimated directly with the physical fractionator method (Gundersen, 1986, Mayhew and Olsen, 1991). This method consists of distribution from counting fields which are systematically and evenly displaced (in a SURS way) on the whole nerve cross-section. Therefore, the frames cover a fixed fraction which is the cross-sectional area one (asf).
| asf = a(frame) / a (section) |
Where: asf is the fraction of area; a(frame) is the area of unbiased counting frame used for quantification of myelinated axonal profiles and, a(sectional) is the distance (ΔX ΔY) between the quantification regions. In this work, the asf was approximately 1/10 (0,11); thus, the average area of counting frame a(frame) was 169,9 µm2 and a(sectional) was 1452 µm2.
Subsequently, the following formula estimates the total number of myelinated axons:
N(axons) = ΣQ- . asf−1.
Where: Σ is the number of axonal profiles sampled (Q-) with the unbiased counting frame multiplied by the inverse of the area fraction, generating an unbiased estimate of the total number of axons (Raimondo et al., 2009).
Perimeter of myelinated axons (b)
The axonal perimeter estimate was obtained with a test system of parallel and isotropic uniform random (IUR) lines overlapping the axonal profile. Counting the number of intersections, I, between the edge of the profile and the test line obtained the estimative of the perimeter (b) of axonal profile which was calculated as π/2 multiplied by the product of the separation of lines (d) and number of intersections between the isotropic lines and inner edge of axonal profile (I) as described in the following formula (Gundersen et al., 1988).
b = π/2. d. I.
Mean thickness of myelin sheath (tmyelin)
The points of intersection between the axon perimeter and the isotropic lines of the test system (the same used for the perimeter estimation) were used to obtain regions in a systematic, uniform, and random way of myelin sheath, and the measurements of its thickness were performed (the result of the first mean of two points per axon and after all axons measured) in approximately one hundred axons (Gundersen et al., 1988, Raimondo et al., 2009).
Statistical analyses
The statistical analysis was performed in Jamovi software version 1.0. Student T test was used for the continuous variable when normality and homogeneity were achieved. In the absence of prerequisites, Mann-Whitney test was used. For categorical variables, Chi-square test was used. Data was derived from another study, complying with reduction and reutilization principles of animal care. Thus, a post-hoc power analysis was performed for estimation of power. The power obtained was 0.36 considering an alpha of 0.05 and an overall effect size of 0.8 (G*Power 3.1.9.7). The significance was considered only when p < 0.05.
Results
Food and water consumption; body mass variation; alcohol intake and its concentration in the blood
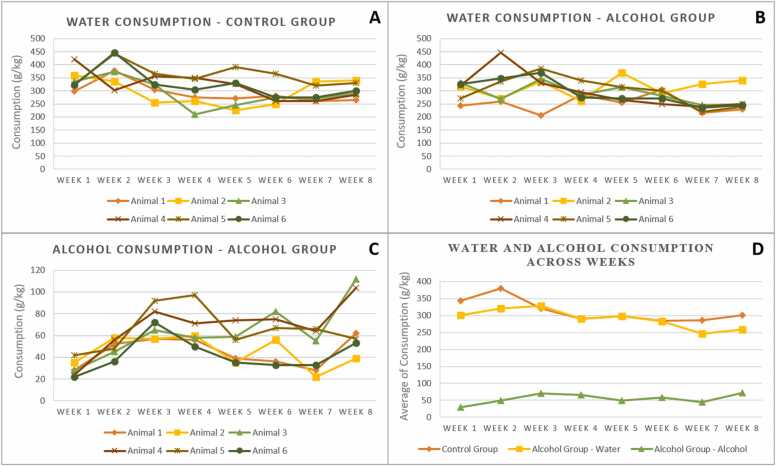
No significant difference between groups was observed in food and water consumption as well as in body mass gain at the end of the treatment, as demonstrated in our previous work (Conte et al., 2019a, Conte et al., 2019b) that used the same animals. In AL group with eight weeks of treatment, the amount of alcohol ingested by the animals and the blood alcohol concentration were also shown in our previous work (mean of 9.86 + 0.84 g / kg / 24 h of alcohol ingested and blood alcohol concentration of 85.1 ± 19.9 mg / dL at the end of the treatment; Conte et al., 2019a, Conte et al., 2019b) where this high concentration was responsible for affecting the brain tissue. Fig. 1 shows the intake profile of alcohol solution and water by the animals of the two groups.
Fig. 1.
- Pattern of water and alcohol consumption between animals of the Control and Alcohol groups. A, water consumption among animals in the Control group; B, water consumption among animals in the Alcohol group; C, alcohol consumption among animals in the Alcohol group; D, water and alcohol average consumption between Control and Alcohol groups.
Tactile and thermal sensitivity tests
In the tactile sensitivity test, there was a significant difference between AL and CO groups (U = 5.50; p = 0.03), being that AL group showed reduction in tactile sensitivity (4.67 ± 1.6) compared to CO group (6.3 ± 0.8). Regarding the thermal sensitivity test, there was no difference between AL and CO groups (U = 8.50; p = 0.14) (Table 1).
Table 1 -.
Tactile and Thermal Sensitivity tests, and Stereological Analysis.
| Control group (n = 6) |
Alcohol group (n = 6) |
p† | |
|---|---|---|---|
| Tactile test [median (interquartile range)] | 4 (4 – 8) | 6 (6 – 8) | 0.04 * |
| Thermal test [median (interquartile range)] | 1.0 (0.6 – 1.7) | 1.22 (0.8 – 6) | 0.14 |
| Stereological Analysis (mean ± SD) | |||
| total number of axons | 1862.17 ± 305.83 | 1680.67 ± 187.9 | 0.24 |
| axonal perimeter (µm) | 958.65 ± 30.9 | 927.12 ± 38.1 | 0.15 |
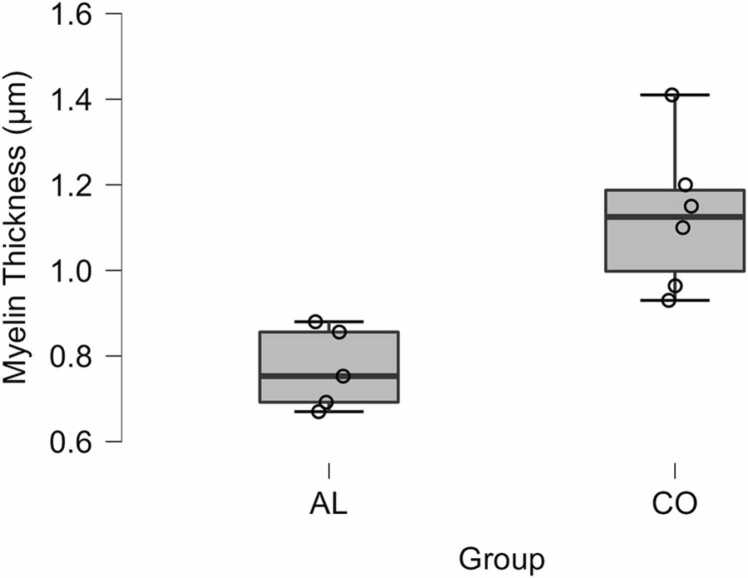
| myelin thickness (µm) | 1.13 ± 0.1 | 0.7 ± 0.08 | 0.001 * * |
† T-student or Mann-Whitney tests. * p < 0.05; * * p < 0.001.
Functional observational battery (FOB)
Neurological domain
Initially, we estimated the MSS of the neurological domain, and the AL group had a score of 9.5 while the CO group had a score ≤ 1. Then, we analyzed each parameter of the neurological domain, evaluating muscle tone, gait, equilibrium, and CNS excitement.
In muscle tone analysis, we evaluated the parameters forelimb grip strength and hypotonia. No difference between AL and CO groups was observed in forelimb grip strength (χ² = 3; p = 0.83) and hypotonia (χ² = 0.01; p = 0.06), respectively. However, 83.3% of animals in the AL group showed excessive resistance to forelimb grip strength (vs 16.7% in the CO group) and 66.7% showed excessive resistance to hypotonia (in the CO group 100% of animals showed normal hypotonia) (Table 2).
Table 2.
Functional Observational Battery (FOB) parameters between of the Control and Alcohol groups.
| Control group (n = 6) |
Alcohol group (n = 6) |
p† | |
|---|---|---|---|
| Neurological Domain | |||
| Forelimb grip strength / Hypotonia (%) | |||
| normal | 83.3 / 100 | 16.7 / 33.3 | 0.83 / 0.06 |
| excessive resistance | 16.7 / 0 | 83.3 / 67.7 | |
| Gait (%) | |||
| normal | 100 | 0 | 0.002 * |
| abnormal | 0 | 100 | |
| Righting reflex (%) | |||
| fall with all four paws | 100 | 0 | 0.002 * |
| all paws do not touch the ground | 0 | 100 | |
| Twitches (%) | |||
| absence | 100 | 0 | 0.004 * |
| marked | 0 | 100 | |
| Clonic convulsions | 100% absence | 100% absence | 1.0 |
| Tonic convulsions | 100% absence | 100% absence | 1.0 |
| Autonomic Domain | |||
| Lacrimation | 100% absence | 100% absence | 1.0 |
| Pupil Size | 100% myosis | 100% myosis | 1.0 |
| Palpebral closure | 100% normal | 100% normal | 1.0 |
| Salivation | 100% absence | 100% absence | 1.0 |
| Breathing | 100% normal | 100% normal | 1.0 |
| Piloerection (%) | |||
| absence | 100 | 0 | 0.002 * |
| moderate | 0 | 66.7 | |
| marked | 0 | 33.3 | |
| Behavioral Domain | |||
| Hyperactivity (%) | |||
| normal | 83.3 | 0 | 0.007 * |
| moderate | 16.7 | 16.7 | |
| high | 0 | 83.3 | |
| Reactivity to Catching / Handling (%) | |||
| normal | 66.7 / 66.7 | 66.7 / 50 | 0.9 / 0.55 |
| little difficulty | 33.3 / 33.3 | 0 / 33.3 | |
| difficulty | 0 / 0 | 0 / 16.7 | |
| Defecation / Urination (%) | |||
| normal | 50 / 66.6 | 33.3 / 66.6 | 0.76 / 0.9 |
| moderate | 33.3 / 16.7 | 33.3 / 16.7 | |
| excessive | 16.7 / 16.7 | 33.3 / 16.7 | |
| Touch response | 100% normal | 100% normal | 1.0 |
| Tail-pinch response | 100% normal | 100% normal | 1.0 |
† Chi-square test. * p < 0.01.
Regarding gait and equilibrium parameters, we observed significant difference in righting reflex between animals of the AL and CO groups (χ² = 0.01; p = 0.002), being that 100% of the animals in the AL group did not fall with their four paws touching the ground (100% of the animals in the CO group fell with their four paws touching the ground). Regarding the gait, we also observed a significant difference between animals of the AL and CO groups (χ² = 0.01; p = 0.002). In this parameter, 100% of the animals in the AL group had a gait pattern considered abnormal (100% of the CO group had a normal gait pattern), since the animals walked on tiptoe and with the belly touching the ground (Table 2).
Finally, within the neurological domain, we evaluated CNS excitement parameters (twitches, clonic and tonic convulsions). For twitches, there was a significant difference between AL and CO groups (χ² = 0.01; p = 0.004), with 100% of the animals in the AL group having marked twitches (100% of the animals in the CO group had the absence of abnormal twitches). Finally, 100% of the animals in both groups had absent clonic or tonic seizures. Therefore, there was no significant difference between the groups (Table 2).
Autonomic domain
For the autonomic domain, MSS score for the AL group was 2.3, while for the CO group it was ≤ 1.
Analyzing each parameter of this domain, we observed a significant difference between AL and CO groups only for piloerection (χ² = 12; p = 0.002). The animals in the AL group had moderate (66.7%) and marked (33.3%) piloerection, mainly around the head and back. Animals in the CO group had no piloerection (0%).
Regarding the other parameters, lacrimation, pupil reflex, palpebral closure, salivation and breathing, there was no significant difference between animals. In both groups AL and CO, the animals showed absence of salivation and lacrimation, normal breathing and palpebral closure, and pupil size with myosis (Table 2).
Behavior domain
The MSS score observed for the behavioral domain was 4.3 in the AL group and ≤ 1.5 in the CO group. Regarding its parameters, we analyzed spontaneous activity, affective response, and sensory responses.
In the spontaneous activity, we analyzed hyperactivity and observed that 83.3% of the animals in the AL group presented high hyperactivity and 16.7% moderate. In the CO group, 83.3% of the animals presented hyperactivity considered normal and 16.7% moderate. Thus, the animals of the AL group presented more active exploratory behavior in the open field (χ² = 10; p = 0.007), demonstrating higher amount of rearing and grooming and reflecting more spontaneous activity (Table 2).
In affective responses, regarding the parameter reactivity to catching (χ² = 1; p = 0.9), reactivity to handling (χ² = 0.343; p = 0.55), defecation (χ² = 0.53; p = 0.76) and urination (χ² = 0.01; p = 0.9), there was no significant difference between AL and CO groups. In the reactivity to catching, 66.7% of the animals in both groups had normal catching behaviour reaction. Regarding reactivity to handling, in 50% of the animals in the AL group the handling difficulty was normal and in 33.3% was with little difficulty (CO group showed that in 66.7% the reactivity to handling was normal and in 33.3% was with little difficulty). Regarding defecation, 33.3% of the animals in the AL group had the amount of defecation considered normal, 33.3% moderate and 33.3% excessive (in the CO group 50% had the amount of defecation normal, 33.3% moderate and 16.7% excessive). Finally, for urination in both groups, 66.6% of the animals had normal volume, 16.7% moderate volume, and 16.7% excessive volume (Table 2).
Sensory tests showed no difference for both touch and tail-pinch response parameters (Table 2).
Stereological analysis
There were no differences between the total number of axons (t (10) = 1.24; p = 0.24) and the axonal perimeter (t (10) = 1.55; p = 0.15) between the animals of the CO and AL groups respectively (Table 1).
However, a significant decrease in the myelin sheath thickness of the animals of the AL group was observed in comparison with the CO group (t (10) = 4.40; p = 0.001) (Fig. 2, Fig. 3). Furthermore, we observed a significant negative correlation (rho = - 0.799; p = 0.002) between alcohol consumption and myelin sheath thickness. There was no significant difference in the correlation between the average alcohol consumption, total number of axons and axonal perimeter.
Fig. 2.
Reduction of the myelin sheath thickness between control and alcohol animals. (A) Control, (B) Alcohol. Scale bar: 20 µm.
Fig. 3.
Myelin sheath thickness between the animals of the Control group (CO) and Alcohol group (AL).
Discussion
In this study, we observed that Wistar rats that consumed alcoholic solution (20%; v/v) for eight weeks showed initial signs of demyelinating lesions in the peripheral nervous system. Based on stereological and functional analyzes (FOB, tactile and thermal tests), we found a significant decrease in the thickness of myelin sheath, alterations in gait and equilibrium (righting reflex), greater tone in lower limbs (twitches), higher amount of rearing and grooming reflecting hyperactivity and piloerection. Furthermore, based on the mean severity score (MSS) of FOB, dysfunctions in the AL group in neurological, autonomic, and behavioral domains over untreated animals were also shown. Additionally, the tactile sensitivity reflex was observed in thinner monofilaments in the AL group.
We observed high consumption of alcohol as expected using Two Bottle Choice Paradigm in animal studies (Wayner et al., 1972, Wise, 1973, O’Dell et al., 2004). Worth mentioning, bottles were tested for leakage, an issue that could hinder precise measurement of the solutions. The leakage was negligible, hence, not affecting the calculations of consumption. Also, levels of alcohol in the blood higher than 60 mg/dL confirmed the consistency of this protocol and were compatible with other studies (Bell et al., 2006, Simms et al., 2008a, Simms et al., 2008b). According to Simms et al., 2008a, Simms et al., 2008b, using similar protocol of intermittent alcohol (20%; v/v) ingestion, the alcohol concentration in the blood ranged from 4 to 93 mg / dL in Wistar rats submitted to 20 ingestion sessions. Bell and collaborators (2006), instead, mentioned that animals prone to alcohol consumption should achieve, at least, 5 g/kg/day of alcohol intake, achieving a concentration of 50–200 mg of alcohol per decilitre of blood (Bell et al., 2006, Simms et al., 2008a, Simms et al., 2008b). An important review on alcohol administration protocols and consumption profiles in animal models, showed that 5–10 g / kg / 24 h per rat or 12–20 g / kg / 24 h per mouse are considered high consumption (in our study we observed a consumption of 9.86 g / kg / 24 h) (Leeman et al., 2010). According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA), acute intoxication in alcohol consumption (binge drinking) corresponds to a blood concentration of 80 mg / dL or higher, and a pattern of heavy drinking to a concentration of 60 mg / dL or higher. In our study, we observed a blood concentration of 85 mg / dL in the AL group, associating the signs and symptoms of AN observed in this study with this pattern of human consumption (NIAAA, 2022). Moreover, because it is a voluntary administration type of methodology, this paradigm mimics human consumption patterns avoiding the stressful effects of invasive and forced administration (Carnicella et al., 2014), that could induce neuroendocrine stress responses and consequently tissue degeneration (Simms et al., 2008a, Simms et al., 2008b, Ferrari et al., 2013).
To unbiasedly estimate the number and diameter of axons of the myelin sheath from a small sample of sciatic nerve, the Optical Fractionator workflow was used (West, 2012, West, 2013). The AL group showed a pattern of demyelination that corroborates with data observed in hyperalgesic and allodynic conditions. It has been suggested that the shared symptomology of these conditions is a consequence of high-frequency ectopic bursts generated on demyelinated Aβ fibers (Mellion et al., 2014, Truini and Cruccu, 2016). However, the absence of significant differences in the axonal number and perimeter could be related to temporal range of alcohol consumption adopted in our protocol (intermittent), since long-term chronic alcoholic patients showed axonal loss (Ballantyne et al., 1980). However, a study carried out by Nguyen et al. (2012), also analyzed the effects of 8 weeks of administration of alcoholic solution (but in concentration of 37%) and the development of AN of Long Evans rats. The study included stereological analysis of nerve and muscle fibers, electrophysiological analysis of nerve conduction, and analysis of the combined effects of insulin/IGF resistance and oxidative stress. The results showed that AN was associated with lower nerve conduction velocity in motor but not sensory fibers, with demyelination and small decrease in axonal diameter with alterations in the cytoskeleton, associated with impaired trophic factor signalling due to insulin and IGF resistance. In this case, the alcohol concentration in the solution was practically double that of our protocol and predominantly motor alterations were observed. Furthermore, a study by Guo et al. (2021) showed that chronic alcohol consumption (5% or 10% for 3 weeks) was associated with white matter atrophy, with a reduction in the number of new oligodendrocytes in the prefrontal cortex and corpus callosum, suggesting that alcohol exposure may inhibit the differentiation of these glial cells and consequently the maintenance of the myelin sheath.
Through global analysis of sensory, motor and autonomic domains by FOB, the MSS scores showed the effect of the substance in the AL group in each of the domains analyzed. Nevertheless, Thermal Sensitivity test showed no significant difference between AL and CO groups. Study carried out by Dina et al. (2000), analyzed AN in Sprague-Dawley rats with alcohol solution consumption for 12 weeks (6.5% concentration). The study evaluated the nociceptive flex reflex, response to Von Frey filaments, thermal and electrophysiological tests, in addition to immunohistochemical study of dorsal ganglia (PKCe activity). Thermal hyperalgesia was observed, in addition to mechanical hyperalgesia and allodynia, no difference in C-fiber conduction velocity, and increased signaling mediated by PKCe. In this study, the alcohol concentration in the solution was much lower than that used by us, but with similar results to ours in terms of sensitivity. Additionally, findings of Fu et al. (2015), with the same alcohol administration protocol used in our study (8–12 weeks) in Sprague-Dawley rats, observed thermal hypersensitivity that further increased for more than seven days after ethanol withdrawal. However, this study tested thermal sensitivity with the use of heat. Study with humans carried out by Hilz et al. (1995), analyzed 50 individuals with alcohol abuse for at least seven years, and the consequent changes in thermal sensitivity, both to cold and heat. The study demonstrated the clinical relevance of thermal sensitivity test, as it is more sensitive for the diagnosis of AN than clinical and nerve conduction velocity tests. In animal studies, the scientific evidence from thermal sensitivity, together with our results from FOB and Von Frey monofilaments, show that chronic alcohol consumption is associated with sensory changes. However, the analysis of these changes is difficult to assess, since it depends on the profile of alcohol consumption and its temporal progression, the administration protocol used and the blood availability of alcohol achieved in each species.
Von Frey monofilaments Tactile Test complemented the FOB data. Our results can represent possible indications of allodynia and hyperalgesia (Jensen and Finnerup, 2014) since animals from AL group presented greater paw withdrawal reflex in response to finer filaments and exacerbated reaction with jumping and vocalization responses. Allodynia and hyperalgesia have been associated with alcohol consumption, as previously described (Fröhlich et al., 2006, Gureje, 2008, Dina et al., 2000). In our study, hyperalgesia can be shown by tactile test, piloerection, alterations in gait pattern and righting reflex, and these observations were taken during FOB, in the AL group. Such gait alterations, although only assessed qualitatively, combined with sensory alterations, are in accordance with data reported by Gatch and Lai with neuropathic humans (Gatch and Lal, 1999). Hyperalgesia is also related to negative emotional states such as depression and anxiety, which are also observed during drug abuse and withdrawal (Valdez et al., 2003, Roberto et al., 2010) and is a predictive factor of pain severity after severe injury (Castillo et al., 2006, Holmes et al., 2010). Thus, hyperalgesia could be explained by the negative emotional state associated with alcohol abuse and by its peripheral effects, especially in long-term consumption and withdrawal phases. (Franklin, 1998, Jochum et al., 2010). A review from Egli et al. (2012) described a central and peripheral sensitization pattern of hyperalgesia in chronic alcohol users. Central sensitization is related to functional alteration in ascending afferent pathways, from dorsal horn of the spinal cord to several limbic structures such as central amygdala and prefrontal cortex (Woolf, 1983, Vogt, 2005, Neugebauer et al., 2009). Reichling and Levine associated peripheral sensitization with the neuroplasticity of peripheral nociceptors (Reichling and Levine, 2009).
A significant difference in the rearing behavior by the AL group in the open field arena was observed. This effect is thought to occur from the action of alcohol on brain areas, such as the nucleus accumbens, which is a region associated with voluntary motor behavior (Boerngen-Lacerda and Souza-Formigoni, 2000). Nucleus accumbens is affected by alcohol consumption culminating in increased locomotor activity due to the sensitization of its neurons (Koob, 1992, Boerngen-Lacerda and Souza-Formigoni, 2000, Koob and Volkow, 2010). Bowen et al. (1996), tested the effect of inhalants solvents to compare with the effect of vapor and intraperitoneal alcohol using the functional observational battery (FOB) and observed that alcohol produces effects on the CNS similar to solvents such as 1,1,1-trichloroethane, ether and flurothyl.
Regarding the autonomic domain, our findings were just significant for piloerection. However, data from literature indicate additional autonomic alterations in long-term chronic users (Milovanovic et al., 2009). One of the tests was 24 h Holter monitoring, which evaluated short-term heart rate variability, showing a significant difference and an incidence of severe autonomic dysfunction in 56% of the patients (chronic alcohol users), (Milovanovic et al., 2009). These studies correlated the autonomic alterations with the total alcohol dose and the number of doses multiplied by the consumption period (El-Mas and Abdel-Rahman, 2013; de Zambotti et al., 2015). In our study, it was not possible to detect all autonomic alterations because the consumption period was not long enough to induce these types of alterations, which should occur after a consumption of alcohol greater than those observed in our animals.
Our results associated with motor functions should be considered with caution, as alcohol itself disrupts motor behavior. Biased results are almost unavoidable in these study models, and an alternative would be to test during drug withdrawal, which would also involve other caveats.
Individual factors also cannot be disregarded. In a study by Mellion et al. (2013) with three different strains of rats, they investigated the effects of alcohol exposure on nerves and muscles. This phenomenon varied according to different susceptibility of genetic patterns, suggesting that nerve injury is influenced by individual genetic characteristics, in addition to chronicity and ingested volume of alcohol (Goldman et al., 2005, Mellion et al., 2013).
Finally, our data indicated the presence of the first symptoms of hyperalgesia, such as increased sensitivity to tactile stimulus, and the stereological analysis showed decrease of the myelin sheath thickness, showing that alcohol ingestion is associated with demyelination process during the hyperalgesia. Some studies about nerve conduction, showing a decrease in nerve impulse speed, associated this effect to a reduction in the number of functional axons (Chopra and Tiwari, 2012). Other studies showing possible demyelination as a consequence of alcohol intake (Mellion et al., 2011) are in agreement with our findings. This show that neuropathic condition is a complex process involving neurons and glial cells.
Although it is still unclear what the pathogenetic mechanisms for the development of neuropathy amongst those who chronically abuse alcohol are, it would seem reasonable to infer that AN is the result of a multifactorial process primarily mediated by the direct toxic effect of alcohol (Mellion et al., 2011).
To date there is no effective therapeutical approach for treating AN, hence the need to explore possible mechanisms involved in alcohol-induced neuropathy so that new therapeutic approaches can be developed for prevention as well as clinical management of this condition (Chopra and Tiwari, 2011). While the data herein presented can contribute to this collective effort, there are limitations worth mentioning. The absence of evaluation of biochemical indicators regarding toxicity in nervous system that could better explain the alcohol-induced neurodegeneration process, what we intend to perform in future studies. In this phase of our study we used male rats, but it is our intention to analyze this process with females as well, providing the oestrous cycle variable can be included. Furthermore, we intend to compare the toxic effect of alcohol on pain central processing to better understand the association between chronic alcohol intake and alcohol-induced neuropathy.
Our data showed a peripheral effect of chronic alcohol intake associated with hyperalgesia and a process of demyelination with a strong correlation with alcohol consumption. This process was associated with increased tactile sensitivity, with behavioral reflexes such as locomotor hyperactivity, changes in gait and balance, and autonomic reflexes such as piloerection.
Financial support
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001, and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brasil – Grant 2019-25423-8 / RTI.
The present work was carried out at Centro de Microscopia Eletrônica, Escola Paulista de Medicina (Universidade Federal de São Paulo; São Paulo, SP, Brazil).
CRediT authorship contribution statement
Maria Eduarda Tessitore= idealization of the proposal, execution of experimental activities, write of manuscript. Laís da Silva Pereira-Rufino= statistical analysis and manuscript review. Carlos Eduardo Panfilio= idealization of the proposal and manuscript review. Rita de Cassia Sinigaglia= supervision of stereological analysis. Odair Aguiar Júnior= provided infrastructure for stereological analysis. Luciana Le-Sueur Maluf= blood alcohol concentration measurement and FOB analysis. Rafael Conte= execution of experimental activities. Fernando Vagner Lobo Ladd= supervision of stereological analysis. Isabel Cristina Céspedes= idealization, general guidance, interpretation of tests and stereological analysis, supply of materials, review of the manuscript.
Conflict of Interest
No conflict of interest.
References
- Ballantyne J.P., Hansen S., Weir A., Whitehead J.R., Mullin P.J. Quantitative electrophysiological study of alcoholic neuropathy. J. Neurol. Neurosurg. Psychiatry. 1980;43:427–432. doi: 10.1136/jnnp.43.5.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behse F., Buchthal F. Peroneal muscular atrophy (PMA) and related disorders. II. Histological findings in sural nerves. Brain. 1977;100(Pt 1):67–85. doi: 10.1093/brain/100.1.67. [DOI] [PubMed] [Google Scholar]
- Bell R.L., Rodd Z.A., Lumeng L., Murphy J.M., McBride W.J. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict. Biol. 2006 doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Boerngen-Lacerda R., Souza-Formigoni M.L.O. Does the increase in locomotion induced by ethanol indicate its stimulant or anxiolytic properties? Pharmacol. Biochem. Behav. 2000 doi: 10.1016/S0091-3057(00)00360-9. [DOI] [PubMed] [Google Scholar]
- Boucard A., Bétat A.M., Forster R., Simonnard A., Froget G. Evaluation of neurotoxicity potential in rats: the functional observational battery. Curr. Protoc. Pharmacol. 2010 doi: 10.1002/0471141755.ph1012s51. [DOI] [PubMed] [Google Scholar]
- Bowen S.E., Wiley J.L., Evans E.B., Tokarz M.E., Balster R.L. Functional observational battery comparing effects of ethanol, 1,1,1-trichloroethane, ether, and flurothyl. Neurotoxicol. Teratol. 1996;18(5):577–585. doi: 10.1016/0892-0362(96)00064-5. [DOI] [PubMed] [Google Scholar]
- Burton R., Sheron N. No level of alcohol consumption improves health. Lancet. 2018;392:987–988. doi: 10.1016/S0140-6736(18)31571-X. [DOI] [PubMed] [Google Scholar]
- Carnicella S., Ron D., Barak S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 2014;48:243–252. doi: 10.1016/j.alcohol.2014.01.006S0741-8329(14)00049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo R.C., MacKenzie E.J., Wegener S.T., Bosse M.J. Prevalence of chronic pain seven years following limb threatening lower extremity trauma. Pain. 2006 doi: 10.1016/j.pain.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Chopra Kanwaljit, Tiwari V. Alcoholic neuropathy: possible mechanisms and future treatment possibilities. Br. J. Clin. Pharm. 2012;73:348–362. doi: 10.1111/j.1365-2125.2011.04111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra K., Tiwari V. Alcoholic neuropathy: possible mechanisms and future treatment possibilities. Br. J. Clin. Pharm. 2012;73:348–362. doi: 10.1111/j.1365-2125.2011.04111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte R., Ladd F.V.L., Ladd A.A.B.L., Moreira A.L., Sueur-Maluf L.L., Viana M.D.B., Céspedes I.C. Behavioral and stereological analysis of the prefrontal cortex of rats submitted to chronic alcohol intake. Behav. Brain Res. 2019:362. doi: 10.1016/j.bbr.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Conte Rafael, Ladd F.V.L., Ladd A.A.B.L., Moreira A.L., Sueur-Maluf L., Le, Viana M., de B., Céspedes I.C. Behavioral and stereological analysis of the prefrontal cortex of rats submitted to chronic alcohol intake. Behav. Brain Res. 2019;362:21–27. doi: 10.1016/j.bbr.2019.01.003. [DOI] [PubMed] [Google Scholar]
- de Zambotti M., Willoughby A.R., Baker F.C., Sugarbaker D.S., Colrain I.M. Cardiac autonomic function during sleep: effects of alcohol dependence and evidence of partial recovery with abstinence. Alcohol. 2015;49:409–415. doi: 10.1016/j.alcohol.2014.07.023S0741-8329(14)20161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina O.A., Barletta J., Chen X., Mutero A., Martin A., Messing R.O., Levine J.D. Key role for the epsilon isoform of protein kinase C in painful alcoholic neuropathy in the rat. J. Neurosci. 2000;20(22):8614–8619. doi: 10.1523/JNEUROSCI.20-22-08614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnadieu-Rigole H., Daien V., Blanc D., Michau S., Villain M., Nalpas B., Perney P. The prevalence of optic neuropathy in alcoholic patients-A pilot study. Alcohol. Clin. Exp. Res. 2014 doi: 10.1111/acer.12468. [DOI] [PubMed] [Google Scholar]
- Duehmke R.M., Derry S., Wiffen P.J., Bell R.F., Aldington D., Moore R.A. Tramadol for neuropathic pain in adults. Cochrane Database Syst. Rev. 2017 doi: 10.1002/14651858.CD003726.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M., Koob G.F., Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci. Biobehav. Rev. 2012 doi: 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas M.M., Abdel-Rahman A.A. Cardiovascular autonomic modulation by nitric oxide synthases accounts for the augmented enalapril-evoked hypotension in ethanol-fed female rats. Alcohol. 2013;47:339–346. doi: 10.1016/j.alcohol.2013.03.004S0741-8329(13)00049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari L.F., Levine E., Levine J.D. Independent contributions of alcohol and stress axis hormones to painful peripheral neuropathy. Neuroscience. 2013;228:409–417. doi: 10.1016/j.neuroscience.2012.10.052S0306-4522(12)01076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin K.B. Analgesia and abuse potential: an accidental association or a common substrate? Pharm. Biochem Behav. 1998;59:993–1002. doi: 10.1016/s0091-3057(97)00535-2. https://doi.org/S0091305797005352 [pii] [DOI] [PubMed] [Google Scholar]
- Fröhlich C., Jacobi F., Wittchen H.U. DSM-IV pain disorder in the general population: an exploration of the structure and threshold of medically unexplained pain symptoms. Eur. Arch. Psychiatry Clin. Neurosci. 2006 doi: 10.1007/s00406-005-0625-3. [DOI] [PubMed] [Google Scholar]
- Fu, R., Gregor, D., Peng, Z., Li, J., Bekker, A., Ye, J.H., 2015. Chronic intermittent voluntary alcohol drinking induces hyperalgesia in Sprague-Dawley rats. Int. J. Physiol. Pathophysiol. Pharmacol. [PMC free article] [PubMed]
- Gatch M.B., Lal H. Effects of ethanol and ethanol withdrawal on nociception in rats. Alcohol. Clin. Exp. Res. 1999 doi: 10.1111/j.1530-0277.1999.tb04118.x. [DOI] [PubMed] [Google Scholar]
- Goldman D., Oroszi G., Ducci F. The genetics of addictions: uncovering the genes. Nat. Rev. Genet. 2005;6:521–532. doi: 10.1038/nrg1635. https://doi.org/nrg1635 [pii]10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Griswold M.G., Fullman N., Hawley C., Arian N., Zimsen S.R.M., Tymeson H.D., Venkateswaran V., Tapp A.D., Forouzanfar M.H., Salama J.S., Abate K.H., Abate D., Abay S.M., Abbafati C., Abdulkader R.S., Abebe Z., Aboyans V., Abrar M.M., Acharya P., Adetokunboh O.O., Adhikari T.B., Adsuar J.C., Afarideh M., Agardh E.E., Agarwal G., Aghayan S.A., Agrawal S., Ahmed M.B., Akibu M., Akinyemiju T., Akseer N., Asfoor D.H., Al, Al-Aly Z., Alahdab F., Alam K., Albujeer A., Alene K.A., Ali R., Ali S.D., Alijanzadeh M., Aljunid S.M., Alkerwi A., Allebeck P., Alvis-Guzman N., Amare A.T., Aminde L.N., Ammar W., Amoako Y.A., Amul G.G.H., Andrei C.L., Angus C., Ansha M.G., Antonio C.A.T., Aremu O., Ärnlöv J., Artaman A., Aryal K.K., Assadi R., Ausloos M., Avila-Burgos L., Avokpaho E.F., Awasthi A., Ayele H.T., Ayer R., Ayuk T.B., Azzopardi P.S., Badali H., Badawi A., Banach M., Barker-Collo S.L., Barrero L.H., Basaleem H., Baye E., Bazargan-Hejazi S., Bedi N., Béjot Y., Belachew A.B., Belay S.A., Bennett D.A., Bensenor I.M., Bernabe E., Bernstein R.S., Beyene A.S., Beyranvand T., Bhaumik S., Bhutta Z.A., Biadgo B., Bijani A., Bililign N., Birlik S.M., Birungi C., Bizuneh H., Bjerregaard P., Bjørge T., Borges G., Bosetti C., Boufous S., Bragazzi N.L., Brenner H., Butt Z.A., Cahuana-Hurtado L., Calabria B., Campos-Nonato I.R., Campuzano J.C., Carreras G., Carrero J.J., Carvalho F., Castañeda-Orjuela C.A., Castillo Rivas J., Catalá-López F., Chang J.-C., Charlson F.J., Chattopadhyay A., Chaturvedi P., Chowdhury R., Christopher D.J., Chung S.-C., Ciobanu L.G., Claro R.M., Conti S., Cousin E., Criqui M.H., Dachew B.A., Dargan P.I., Daryani A., Das Neves J., Davletov K., De Castro F., De Courten B., De Neve J.-W., Degenhardt L., Demoz G.T., Des Jarlais D.C., Dey S., Dhaliwal R.S., Dharmaratne S.D., Dhimal M., Doku D.T., Doyle K.E., Dubey M., Dubljanin E., Duncan B.B., Ebrahimi H., Edessa D., El Sayed Zaki M., Ermakov S.P., Erskine H.E., Esteghamati A., Faramarzi M., Farioli A., Faro A., Farvid M.S., Farzadfar F., Feigin V.L., Felisbino-Mendes M.S., Fernandes E., Ferrari A.J., Ferri C.P., Fijabi D.O., Filip I., Finger J.D., Fischer F., Flaxman A.D., Franklin R.C., Futran N.D., Gallus S., Ganji M., Gankpe F.G., Gebregergs G.B., Gebrehiwot T.T., Geleijnse J.M., Ghadimi R., Ghandour L.A., Ghimire M., Gill P.S., Ginawi I.A., Giref A.Z.Z., Gona P.N., Gopalani S.V., Gotay C.C., Goulart A.C., Greaves F., Grosso G., Guo Y., Gupta Rahul, Gupta Rajeev, Gupta V., Gutiérrez R.A., GVS M., Hafezi-Nejad N., Hagos T.B., Hailu G.B., Hamadeh R.R., Hamidi S., Hankey G.J., Harb H.L., Harikrishnan S., Haro J.M., Hassen H.Y., Havmoeller R., Hay S.I., Heibati B., Henok A., Heredia-Pi I., Hernández-Llanes N.F., Herteliu C., Hibstu D.T.T., Hoogar P., Horita N., Hosgood H.D., Hosseini M., Hostiuc M., Hu G., Huang H., Husseini A., Idrisov B., Ileanu B.V., Ilesanmi O.S., Irvani S.S.N., Islam S.M.S., Jackson M.D., Jakovljevic M., Jalu M.T., Jayatilleke A.U., Jha R.P., Jonas J.B., Jozwiak J.J., Kabir Z., Kadel R., Kahsay A., Kapil U., Kasaeian A., Kassa T.D.D., Katikireddi S.V., Kawakami N., Kebede S., Kefale A.T., Keiyoro P.N., Kengne A.P., Khader Y., Khafaie M.A., Khalil I.A., Khan M.N., Khang Y.-H., Khater M.M., Khubchandani J., Kim C.-I., Kim D., Kim Y.J., Kimokoti R.W., Kisa A., Kivimäki M., Kochhar S., Kosen S., Koul P.A., Koyanagi A., Krishan K., Kuate Defo B., Kucuk Bicer B., Kulkarni V.S., Kumar P., Lafranconi A., Lakshmana Balaji A., Lalloo R., Lallukka T., Lam H., Lami F.H., Lan Q., Lang J.J., Lansky S., Larsson A.O., Latifi A., Leasher J.L., Lee P.H., Leigh J., Leinsalu M., Leung J., Levi M., Li Y., Lim L.-L., Linn S., Liu S., Lobato-Cordero A., Lopez A.D., Lotufo P.A., Macarayan E.R.K., Machado I.E., Madotto F., Magdy Abd El Razek H., Magdy Abd El Razek M., Majdan M., Majdzadeh R., Majeed A., Malekzadeh R., Malta D.C., Mapoma C.C., Martinez-Raga J., Maulik P.K., Mazidi M., Mckee M., Mehta V., Meier T., Mekonen T., Meles K.G., Melese A., Memiah P.T.N., Mendoza W., Mengistu D.T., Mensah G.A., Meretoja T.J., Mezgebe H.B., Miazgowski T., Miller T.R., Mini G., Mirica A., Mirrakhimov E.M., Moazen B., Mohammad K.A., Mohammadifard N., Mohammed S., Monasta L., Moraga P., Morawska L., Mousavi S.M., Mukhopadhyay S., Musa K.I., Naheed A., Naik G., Najafi F., Nangia V., Nansseu J.R., Nayak M.S.D.P., Nejjari C., Neupane S., Neupane S.P., Ngunjiri J.W., Nguyen C.T., Nguyen L.H., Nguyen T.H., Ningrum D.N.A., Nirayo Y.L., Noubiap J.J., Ofori-Asenso R., Ogbo F.A., Oh I.-H., Oladimeji O., Olagunju A.T., Olivares P.R., Olusanya B.O., Olusanya J.O., Oommen A.M., Oren E., Orpana H.M., Ortega-Altamirano D.D.V., Ortiz J.R., Ota E., Owolabi M.O., Oyekale A.S., P.A., M., Pana A., Park E.-K., Parry C.D.H., Parsian H., Patle A., Patton G.C., Paudel D., Petzold M., Phillips M.R., Pillay J.D., Postma M.J., Pourmalek F., Prabhakaran D., Qorbani M., Radfar A., Rafay A., Rafiei A., Rahim F., Rahimi-Movaghar A., Rahman M., Rahman M.A., Rai R.K., Rajsic S., Raju S.B., Ram U., Rana S.M., Ranabhat C.L., Rawaf D.L., Rawaf S., Reiner R.C., Reis C., Renzaho A.M.N., Rezai M.S., Roever L., Ronfani L., Room R., Roshandel G., Rostami A., Roth G.A., Roy A., Sabde Y.D., Saddik B., Safiri S., Sahebkar A., Salama J.S., Saleem Z., Salomon J.A., Salvi S.S., Sanabria J., Sanchez-Niño M.D., Santomauro D.F., Santos I.S., Santric Milicevic M.M.M., Sarker A.R., Sarmiento-Suárez R., Sarrafzadegan N., Sartorius B., Satpathy M., Sawhney M., Saxena S., Saylan M., Schaub M.P., Schmidt M.I., Schneider I.J.C., Schöttker B., Schutte A.E., Schwendicke F., Sepanlou S.G., Shaikh M.A., Sharif M., She J., Sheikh A., Shen J., Shiferaw M.S., Shigematsu M., Shiri R., Shishani K., Shiue I., Shukla S.R., Sigfusdottir I.D., Silva D.A.S., Silva N.T.Da, Silveira D.G.A., Sinha D.N., Sitas F., Soares Filho A.M., Soofi M., Sorensen R.J.D., Soriano J.B., Sreeramareddy C.T., Steckling N., Stein D.J., Sufiyan M.B., Sur P.J., Sykes B.L., Tabarés-Seisdedos R., Tabuchi T., Tavakkoli M., Tehrani-Banihashemi A., Tekle M.G., Thapa S., Thomas N., Topor-Madry R., Topouzis F., Tran B.X., Troeger C.E., Truelsen T.C., Tsilimparis N., Tyrovolas S., Ukwaja K.N., Ullah I., Uthman O.A., Valdez P.R., Van Boven J.F.M., Vasankari T.J., Venketasubramanian N., Violante F.S., Vladimirov S.K., Vlassov V., Vollset S.E., Vos T., Wagnew F.W.S., Waheed Y., Wang Y.-P., Weiderpass E., Weldegebreal F., Weldegwergs K.G., Werdecker A., Westerman R., Whiteford H.A., Widecka J., Wijeratne T., Wyper G.M.A., Xu G., Yamada T., Yano Y., Ye P., Yimer E.M., Yip P., Yirsaw B.D., Yisma E., Yonemoto N., Yoon S.-J., Yotebieng M., Younis M.Z., Zachariah G., Zaidi Z., Zamani M., Zhang X., Zodpey S., Mokdad A.H., Naghavi M., Murray C.J.L., Gakidou E. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen H.J. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J. Microsc. 1986;143:3–45. doi: 10.1111/j.1365-2818.1986.tb02764.x. [DOI] [PubMed] [Google Scholar]
- Gundersen H.J., Bagger P., Bendtsen T.F., Evans S.M., Korbo L., Marcussen N., Moller A., Nielsen K., Nyengaard J.R., Pakkenberg B., et al. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Guo F., Zhang Y.-F., Liu K., Huang X., Li R.-X., Wang S.-Y., Wang F., Xiao L., Mei1 F., Li T. Exposure to alcohol inhibits new myelin generation in adult mouse brain. Front. Cell. Neurosci. 2021;15 doi: 10.3389/fncel.2021.732602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureje O. Treating chronic pain in the context of comorbid depression. Pain. 2008 doi: 10.1016/j.pain.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Holmes A., Williamson O., Hogg M., Arnold C., Prosser A., Clements J., Konstantatos A., O’Donnell M. Predictors of pain 12 months after serious injury. Pain. Med. 2010;11:1599–1611. doi: 10.1111/j.1526-4637.2010.00955.x. [DOI] [PubMed] [Google Scholar]
- Hwa L.S., Kalinichev M., Haddouk H., Poli S., Miczek K.A. Reduction of excessive alcohol drinking by a novel GABAB receptor positive allosteric modulator ADX71441 in mice. Psychopharmacol. (Berl. ) 2014 doi: 10.1007/s00213-013-3245-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen T.S., Finnerup N.B. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 2014;13:924–935. doi: 10.1016/S1474-4422(14)70102-4. [DOI] [PubMed] [Google Scholar]
- Jochum T., Boettger M.K., Burkhardt C., Juckel G., Bar K.J. Increased pain sensitivity in alcohol withdrawal syndrome. Eur. J. Pain. 2010;14:713–718. doi: 10.1016/j.ejpain.2009.11.008S1090-3801(09)00243-2. [DOI] [PubMed] [Google Scholar]
- Kandhare A.D., Raygude K.S., Ghosh P., Ghule A.E., Bodhankar S.L. Therapeutic role of curcumin in prevention of biochemical and behavioral aberration induced by alcoholic neuropathy in laboratory animals. Neurosci. Lett. 2012 doi: 10.1016/j.neulet.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Kaur M., Singh A., Kumar B., Singh S.K., Bhatia A., Gulati M., Prakash T., Bawa P., Malik A.H. Protective effect of co-administration of curcumin and sildenafil in alcohol induced neuropathy in rats. Eur. J. Pharmacol. 2017 doi: 10.1016/j.ejphar.2017.03.012. [DOI] [PubMed] [Google Scholar]
- Koike H., Mori K., Misu K., Hattori N., Ito H., Hirayama M., Sobue G. Painful alcoholic polyneuropathy with predominant small-fiber loss and normal thiamine status. Neurology. 2001;56:1727–1732. doi: 10.1212/wnl.56.12.1727. [DOI] [PubMed] [Google Scholar]
- Koob G.F. Neural mechanisms of drug reinforcement. Ann. N. Y. Acad. Sci. 1992:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman R.F., Heilig M., Cunningham C.L., Stephens D.N., Duka T., O’Malley S.S. Ethanol Consumption: How Should We Measure It? Achieving Consilience between Human and Animal Phenotypes. Addict. Biol. 2010;15(2):109–124. doi: 10.1111/j.1369-1600.2009.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiya R.P., Messing R.O. Peripheral systems: neuropathy. Handb. Clin. Neurol. 2014 doi: 10.1016/B978-0-444-62619-6.00029-X. [DOI] [PubMed] [Google Scholar]
- Mayhew T.M. A geometric model for estimating villous surface area in rat small bowel is justified by unbiased estimates obtained using vertical sections. J. Anat. 1988;161:187–193. [PMC free article] [PubMed] [Google Scholar]
- Mayhew T.M., Olsen D.R. Magnetic resonance imaging (MRI) and model-free estimates of brain volume determined using the Cavalieri principle. J. Anat. 1991;178:133–144. [PMC free article] [PubMed] [Google Scholar]
- Mellion M., Gilchrist J.M., De La Monte S. Alcohol-related peripheral neuropathy: nutritional, toxic, or both? Muscle Nerve. 2011 doi: 10.1002/mus.21946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellion M.L., Nguyen V., Tong M., Gilchrist J., De La Monte S. Experimental model of alcohol-related peripheral neuropathy. Muscle Nerve. 2013;48:204–211. doi: 10.1002/mus.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellion M.L., Silbermann E., Gilchrist J.M., Machan J.T., Leggio L., de la Monte S. Small-fiber degeneration in alcohol-related peripheral neuropathy. Alcohol. Clin. Exp. Res. 2014 doi: 10.1111/acer.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P., Hargreaves J., Curtis A., Zinkiewicz L. The association between an abusive father-son relationship, quantity of alcohol consumption, and male-to-male alcohol-related aggression. Alcohol. Clin. Exp. Res. 2013;37:1571–1576. doi: 10.1111/acer.12114. [DOI] [PubMed] [Google Scholar]
- Milovanovic B., Milinic N., Trifunovic D., Krotin M., Filipovic B., Bisenic V., Djuric D. Autonomic dysfunction in alcoholic cirrhosis and its relation to sudden cardiac death risk predictors. Gen. Physiol. Biophys. 2009;28:251–261. (Spec No) [PubMed] [Google Scholar]
- NIAAA - National Institute on Alcohol Abuse and Alcoholism USA, 2022. 〈https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking〉 (accessed 12 August 2022).
- Hilz M.J., Zimmermann P., Claus D., Neundorfer B. Thermal threshold determination in alcoholic polyneuropathy: an improvement of diagnosis. Acta Neurol. Scand. 1995;91:389–393. doi: 10.1111/j.1600-0404.1995.tb07026.x. [DOI] [PubMed] [Google Scholar]
- Neugebauer V., Galhardo V., Maione S., Mackey S.C. Forebrain pain mechanisms. Brain Res. Rev. 2009;60:226–242. doi: 10.1016/j.brainresrev.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V.A., Le T., Tong M., Silbermann E., Gundogan F., de la Monte S.M. Impaired insulin/IGF signaling in experimental alcohol-related myopathy. Nutrients. 2012;4:1058–1075. doi: 10.3390/nu4081058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell L.E., Roberts A.J., Smith R.T., Koob G.F. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol. Clin. Exp. Res. 2004;28:1676–1682. doi: 10.1097/01.ALC.0000145781.11923.4E. [DOI] [PubMed] [Google Scholar]
- Pitcher G.M., Ritchie J., Henry J.L. Paw withdrawal threshold in the von Frey hair test is influenced by the surface on which the rat stands. J. Neurosci. Methods. 1999 doi: 10.1016/S0165-0270(99)00004-7. [DOI] [PubMed] [Google Scholar]
- Raimondo S., Fornaro M., Di Scipio F., Ronchi G., Giacobini-Robecchi M.G., Geuna S. Chapter 5: methods and protocols in peripheral nerve regeneration experimental research: part II-morphological techniques. Int.. Rev. Neurobiol. 2009;87:81–103. doi: 10.1016/S0074-7742(09)87005-0S0074-7742(09)87005-0. [DOI] [PubMed] [Google Scholar]
- Reichling D.B., Levine J.D. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M., Cruz M.T., Gilpin N.W., Sabino V., Schweitzer P., Bajo M., Cottone P., Madamba S.G., Stouffer D.G., Zorrilla E.P., Koob G.F., Siggins G.R., Parsons L.H. Corticotropin releasing factor–induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol. Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms J. a, Steensland P., Medina B., Abernathy K.E., Chandler L.J., Wise R., Bartlett S.E. Intermittent access to 20% ethanol induces high ethanol consumption in long-evans and wistar rats. Alcohol. Clin. Exp. Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms J.A., Steensland P., Medina B., Abernathy K.E., Chandler L.J., Wise R., Bartlett S.E. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin. Exp. Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.xACER753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truini A., Cruccu G. How diagnostic tests help to disentangle the mechanisms underlying neuropathic pain symptoms in painful neuropathies. Pain. 2016 doi: 10.1097/j.pain.0000000000000367. [DOI] [PubMed] [Google Scholar]
- Valdez G.R., Zorrilla E.P., Roberts A.J., Koob G.F. High-priority communication I Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol. 2003;29:55–60. doi: 10.1016/S0741-8329(03)00020-X. [DOI] [PubMed] [Google Scholar]
- Vogt B.A. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 2005 doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner M.J., Greenberg I., Tartaglione R., Nolley D., Fraley S., Cott A. A new factor affecting the consumption of ethyl alcohol and other sapid fluids. Physiol. Behav. 1972;8:345–362. doi: 10.1016/0031-9384(72)90383-6. https://doi.org/0031-9384(72)90383-6 [pii] [DOI] [PubMed] [Google Scholar]
- West M.J. Estimating length in biological structures. Cold Spring Harb. Protoc. 2013;2013:412–420. doi: 10.1101/pdb.top071811pdb.top071811. [pii]2013/5/pdb.top071811 [pii] [DOI] [PubMed] [Google Scholar]
- West M.J. Introduction to stereology. Cold Spring Harb. Protoc. 2012:2012. doi: 10.1101/pdb.top070623pdb.top070623. [pii]2012/8/pdb.top070623 [pii] [DOI] [PubMed] [Google Scholar]
- Wise R.A. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973 doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Woolf C.J. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983 doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- World Health Organization Global status report on alcohol and health 2018. Glob. Status Rep. Alcohol. 2018 (https://doi.org//entity/substance_abuse/publications/global_alcohol_report/en/index.html) [Google Scholar]
- Wscieklica T., Le Sueur-Maluf L., Prearo L., Conte R., Viana M., de B., Céspedes I.C. Chronic intermittent ethanol administration differentially alters DeltaFosB immunoreactivity in cortical-limbic structures of rats with high and low alcohol preference. Am. J. Drug Alcohol Abus. 2019:1–12. doi: 10.1080/00952990.2019.1569667. [DOI] [PubMed] [Google Scholar]
- Zambelis T., Karandreas N., Tzavellas E., Kokotis P., Liappas J. Large and small fiber neuropathy in chronic alcohol-dependent subjects. J. Peripher Nerv. Syst. 2005;10:375–381. doi: 10.1111/j.1085-9489.2005.00050.x. https://doi.org/JNS050 [pii] [DOI] [PubMed] [Google Scholar]