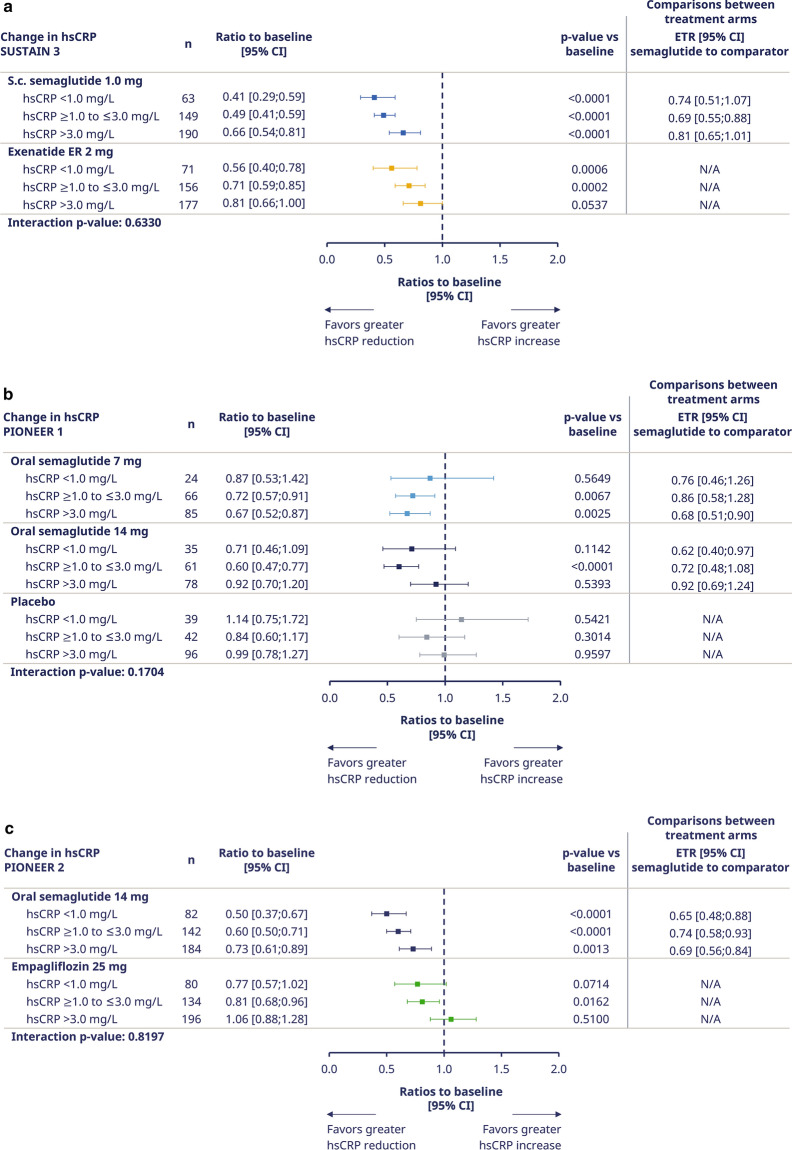
Fig. 2.
Ratio to baseline at end-of-treatment for hsCRP by trial according to clinical cutoffs. Panel (a) shows SUSTAIN 3 data, panel (b) PIONEER 1 data, panel (c) PIONEER 2 data, and panel (d) PIONEER 5 data. ‘On-treatment without rescue medication’ data from the full analysis set. Ratios to baseline were analyzed using a mixed model for repeated measurements with treatment by hsCRP groups as categorical fixed effects and baseline hsCRP value (log-transformed) as covariate, all nested within visit, and an unstructured residual covariance matrix on log-transformed values. Clinical cut-offs used in this analysis were < 1.0, ≥ 1.0 to ≤ 3.0, and > 3.0 mg/L. CI confidence interval; ETR estimated treatment ratio; exenatide ER exenatide extended-release; hsCRP high-sensitivity C-reactive protein; N number of subjects with available hsCRP data; s.c. subcutaneous