Abstract
Global vaccination against the coronavirus disease 2019 (COVID-19) is thought to be the most effective way to end or at least contain the COVID-19 pandemic. However, despite the good safety profiles and effectiveness of the available COVID-19 vaccines, rare but serious hematological complications have emerged, including thromboembolic outcomes with thrombocytopenia following the ChAdOx1 nCoV-19 (Oxford-AstraZeneca) and Ad26.COV.2.S (Johnson & Johnson/Janssen) vaccines. Moreover, COVID-19 vaccination may be linked to the development of aplastic anemia (AA). We discuss four cases of AA that arose after COVID-19 vaccination in our hospital and two other such cases identified in our literature review.
Keywords: COVID-19, Coronavirus disease 2019, SARS-CoV-2, Severe acute respiratory syndrome coronavirus-2 vaccine, Vaccination, Aplastic anemia
Introduction
In December 2019, the first reports emerged of a novel coronavirus disease–subsequently named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, or COVID-19)–that rapidly spread worldwide and became a deadly pandemic. Global vaccination is considered to be the most effective way of ending or at least containing the COVID-19 pandemic. Several COVID-19 vaccines with different mechanisms have been produced and have demonstrated good safety profiles and effectiveness, although there are reports of hematological events. These include several hundred cases of a rare clotting syndrome referred to as vaccine-induced immune thrombotic thrombocytopenia (VITT) emerging after vaccination with the adenoviral-vectored Oxford-AstraZeneca and Johnson & Johnson/Janssen vaccines [1]; severe autoimmune hemolytic anemia and immune thrombocytopenic purpura have also been reported after COVID-19 vaccination [2, 3]. However, aplastic anemia (AA) after COVID-19 vaccination is rarely discussed. Here, we report four cases of AA potentially related to the administration of COVID-19 vaccine, all of which were treated with immunosuppression with or without eltrombopag; the development of severe infection in one case required salvage haploidentical peripheral stem cell transplantation (PBSCT).
Case 1
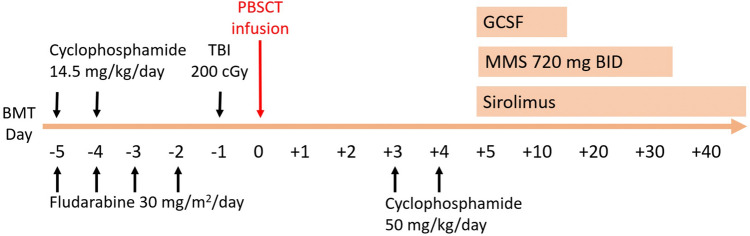
A 64-year-old woman presented to our hospital with a 3-week history of petechiae over the lower limbs, followed by fever for 1 week. She was previously healthy and denied symptoms of cytopenia (i.e., pallor, fatigue, jaundice, bleeding, severe or frequent infections) before COVID-19 vaccination. She received one dose of the Oxford-AstraZeneca (AZ) COVID-19 vaccine on 27 July 2021, soon after the vaccination, she had fever for 1 day. Five days later, some petechiae over lower limbs developed. She visited local hospital due to progressing petechiae 1 week later, and laboratory tests revealed moderate neutropenia (absolute neutrophil count [ANC] 990/μL], thrombocytopenia (platelet count [PLT] 3,000/μL), anemia (hemoglobin [Hb] 7.8 g/dL), then she was transferred to regional hospital where bone marrow examination was suggested, but she refused. Methylprednisolone 80 mg/day and blood transfusion was given, after then her symptoms improved. 10 days later, she came to our hospital due to fever for 1 day. At admission, laboratory tests revealed severe neutropenia (ANC 9.9/μL], thrombocytopenia (PLT < 10,000/μL), normocytic anemia (Hb 10 g/dL, mean corpuscular volume [MCV] 82.2 fl) and decreased reticulocyte count (1422/μL). Hemolytic markers were within normal limits. Blood smear analysis showed no schistocytes or blasts. Evaluation tests for hepatitis B virus (HBV), human immunodeficiency virus (HIV), hepatitis C virus (HCV), cytomegalovirus (CMV) and parvovirus B19 performed by both serology and polymerase chain reaction (PCR) techniques did not show any evidence of active infections. Autoimmune tests for anti-nuclear antibodies (ANA) and anti-double stranded DNA (anti-dsDNA) antibodies were negative. Bone marrow biopsy showed only 0–3% cellularity and marked depletion of normal hematopoietic cells; chromosome analysis could not be performed because of the poor sample quality. The patient was diagnosed with very severe AA (VSAA) on the basis of the modified Camitta criteria. After admission, fungal pneumonia was identified by computed tomography (CT) imaging. After controlling the fungal pneumonia with voriconazole, we started immunosuppressive therapy (IST) plus eltrombopag according to an approved regimen [4] on 30 August 2021. We used the IST including cyclosporin, starting from 3 mg/kg every 12 h to keep serum level between 200 and 400 mcg/L, rabbit antithymocyte globulin (r-ATG) at the dose of 3.5 mg/kg per day for 5 days, prednisolone of 1 mg/kg for 10 days then tapering in the following 5 days, and eltrombopag, at the dose of 50 mg/day(due to financial issue). However, in the third week of IST, the fungal infection progressed with paranasal sinus invasion despite double antifungal drugs (voriconazole and liposomal amphotericin B) and febrile neutropenia persisted. Repeat bone marrow biopsy revealed persistent 0–3% cellularity without progenitor cells. Salvage haploidentical PBSCT was therefore arranged (see Fig. 1 for details of the protocol). The patient experienced uneventful hematological recovery on Day 12 and the chromosome from bone marrow aspiration showed donor-chimerism. On Day 93 (31 December 2021), she had no clinical manifestations of graft-versus-host disease (GVHD) in the skin, gastrointestinal tract, or other target organs.
Fig. 1.
Our conditioning regimen Consisted of fludarabine 30 mg/m2/day for 4 days, cyclophosphamide 14.5 mg/kg/day for 2 days and total body irradiation (TBI) of 200 cGy on day -1, followed by post-transplant cyclophosphamide as graft-versus-host disease prophylaxis
Case 2
A 73-year-old man presented to our hospital with dyspnea on exertion that had developed 2 weeks after receiving his second dose of the Moderna messenger RNA (mRNA) COVID-19 vaccine. He had fever and muscles soreness 1 day after the vaccination. He was a previously healthy farmer and denied any symptoms of cytopenia. Laboratory tests revealed neutropenia (ANC 499/μL), thrombocytopenia (PLT 13,000/μL), normocytic anemia (Hb 7.9 g/dL, MCV 96.3 fl) and decreased reticulocyte count (18,190/μL). Bone marrow biopsy showed only 0–5% cellularity and marked depletion of normal hematopoietic cells; chromosome analysis was normal (karyotype 46,XY). After exclusion of autoimmune disease, HBV, HCV, HIV, or CMV infections and hemolysis, SAA was diagnosed and 1 week later the patient commenced IST with eltrombopag. The dose of eltrombopag was 100 mg/kg per day. The patient’s ANC elevated to more than 1,000/μL on Day 10 of IST administration and he achieved a partial response(ANC 2,214/μL, Hb 7.7 g/dL, PLT 23,000/μL, reticulocyte count 20,331/μL) with transfusion independence on Day 27. On Day 98 of IST, he has been maintained on cyclosporin plus eltrombopag under partial response of SAA (ANC 2477/μL, Hb 9.2 g/dL, PLT 96,000/μL, reticulocyte count 81,144/μL) without neither blood transfusion nor granulocyte colony-stimulating factor supply by attending our Outpatient Department.
Case 3
A 64-year-old man was diagnosed with moderate AA (ANC 747/μL, Hb 6.9 g/dL, PLT 15,000/μL, reticulocyte count 21,800/μL) 2 weeks after receiving the first dose of AZ COVID-19 vaccine. Immediately prior to vaccination, he had normal white blood cells, Hb and PLT values. He did not have fever or other symptoms after vaccination. The bone marrow study showed hypocellularity (3–5%) with normal karyotype. We observed him for 1 month before starting cyclosporin monotherapy. He achieved partial response on day 72, however, on day 118, his platelet count dropped to 18,000/μL. We plan to initiate IST if persistent blood transfusion dependent.
Case 4
Case 4 is a 19-year-old student with a history of thalassemia and a normal complete blood count (CBC) obtained 1 year earlier. He did not have fever or other symptoms after receiving the first dose of the Pfizer-BioNTech (BNT) COVID-19 vaccine. However, three weeks later, he began to experience severe dyspnea on exertion. After undergoing a series of examinations, he was diagnosed as having SAA(ANC 281/μL, Hb 4.3 g/dL, PLT 11,000/μL). The bone marrow study showed hypocellularity (10–15%) with normal karyotype. Over 1 month of observation, recovery did not occur. As the patient had no matched sibling donor, he commenced IST plus eltrombopag. Currently, he achieved partial remission on day 47 after IST started.
Discussion
Acquired AA is a disorder resulting from damage to progenitor cells by chemicals, exposure to ionizing radiation, drugs, viral infections, constitutional genetic defects, or autoimmune destruction. Postvaccination AA has seldom been discussed. Case reports have described acquired SAA developing shortly after HBV, anthrax, or H1N1 influenza vaccination, all involving young men administered human leukocyte antigen (HLA)-haploidentical bone marrow transplantation [5, 6]. Our literature search revealed two cases of SAA following COVID-19 vaccination. One case report described how a previously healthy 76-year-old man developed SAA at 1 month after his second dose of the BNT mRNA COVID-19 vaccine. Hematological parameters did not respond to first-line IST (without eltrombopag) and his condition was complicated by serum sickness and infections [7]. The other case involved a 56-year-old patient who developed VSAA after his second dose of the BNT mRNA COVID-19 vaccine; an allogeneic hematopoietic stem cell transplantation resulted in hematological recovery after 3 weeks [8].
To our knowledge, Case 1 is the first-ever report of VSAA developing after a first dose of AZ COVID-19 vaccine. Although the symptoms of thrombocytopenia was occurred rapidly after This patient had severe fungal infection during IST and eventually experienced recovery after salvage haploidentical PBSCT. Case 2, a patient with SAA occurring after his second dose of the Moderna mRNA COVID-19 vaccine, was also successfully treated with IST plus eltrombopag. Case 3 developed moderate AA after the first dose of the AZ COVID-19 vaccine and responded well to treatment with cyclosporin monotherapy. Case 4 is a 19-year-old male with a history of thalassemia who developed SAA 3 weeks after the first dose of BNT COVID-19 vaccine; he did not have a matched sibling donor so is undergoing IST with eltrombopag, without hematological recovery at the time of this report. Table 1 summarizes the treatment strategies and responses of all four cases in our institute.
Table 1.
Summary of treatment strategies and responses among four patients who developed aplastic anemia after receiving the COVID-19 vaccine and admission to China Medical University Hospital (Taichung, Taiwan)
| Case | Age (years) | Sex | Vaccine | Time to symptom onset | AA type | Treatment | Response |
|---|---|---|---|---|---|---|---|
| 1 | 64 | F | AZ | 5 days | VSAA | IST plus eltrombopag followed by PBSCT | Hematological recovery on day 12 after PBSCT |
| 2 | 73 | M | Moderna | 15 days after 2nd dose | SAA | IST plus eltrombopag | Hematological recovery on day 18 after IST |
| 3 | 64 | M | AZ | 2 weeks | Moderate AA | Cyclosporin | Partial response on day 27 after cyclosporin, but platelet count decreased on day 118 |
| 4 | 19 | M | BNT | 3 weeks | SAA | IST plus eltrombopag | Partial response on day 47 after IST |
Cases 1, 2: No previous complete blood count (CBC) data
Case 3: Had normal CBC before vaccination
Case 4: Had a history of thalassemia. A health check-up 1 year prior to vaccination showed only microcytic anemia
F female, M male, AZ Oxford-AstraZeneca, AA aplastic anemia, VSAA very severe aplastic anemia, SAA severe aplastic anemia, IST immunosuppression therapy, PBSCT peripheral blood stem cell transplantation
The pathogenesis of idiopathic AA, or acquired AA caused by autoimmune-related hematopoietic cell destruction, is characterized by the production of cytotoxic T cells that produce inhibitory cytokines, including interferon gamma (IFN-γ) and tumor necrosis factor, capable of suppressing hematopoiesis by inducing Fas-mediated apoptosis [9]. One study has shown that the AZ COVID-19 vaccine induced a stronger T cell response than after BNT priming, although T cell responses were similarly increased by the second BNT dose [10]. Notably, while both vaccine schedules induced progressive increases in percentages of IFN-γ-positive CD4+ and CD8+ T cells, IFN-γ production was stronger after both the priming and second doses of the AZ vaccine[10] which may mean that this vaccine is more likely than the BNT vaccine to destroy progenitor cells, a mechanism that is similar to that of idiopathic AA and acquired AA. If true, then this phenomenon may support the contention that AA is potentially induced by COVID-19 vaccination and with greater likelihood after the adenoviral-vectored COVID-19 vaccines than after the mRNA vaccines. In reference to our cases, AA occurred after either the adenoviral-vectored or mRNA vaccines, but apparently with greater severity after the adenoviral-vectored vaccine platform. We therefore hypothesize that the AZ vaccine may be more likely to induce aberrant T cell activation than the mRNA vaccine, resulting in a more advanced grade of AA after the first dose, while this reaction may occur only after the second mRNA dose. However, in our Case 4, SAA developed after the first dose of mRNA vaccine. We also speculate that younger age groups may mount a stronger immune response than that observed in older aged individuals, but we need more data to support these thoughts.
Furthermore, whether the addition of eltrombopag improves the treatment response in patients who develop SAA after vaccination is also an issue. It has been conjectured that eltrombopag may enhance the effect of thrombopoietin (TPO) in vivo and thus overcome the suppressive mechanism of IFN-γ upon TPO signaling in hematopoietic stem cells [11]. Interestingly, whereas the case of SAA that developed in a 76-year-old man after BNT mRNA vaccination was not treated with eltrombopag and did not experience significant improvement for 4 months,7 our Case 2 (also a man in his 70 s) achieved a partial response with transfusion independence by Day 27 of treatment with IST plus eltrombopag.
In conclusion, although there is no established causative relationship between COVID-19 vaccination and AA, and consensus is lacking as to ideal treatment regimens for AA developing after COVID-19 vaccination, the experience of our institute offers a viable treatment strategy.
Acknowledgements
We would like to thank Iona MacDonald from China Medical University for her professional editing of this report.
Author contributions
All authors contributed to the conception and design of the study. Cases were provided by T-TC, M-YL, C-YH and C-CC. Collection and assembly of data was performed by C-YC. The first draft of the manuscript was written by C-YC and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported in part by grants from the Ministry of Health and Welfare, Taiwan (MOHW107- TDU-B-212-114026A) and the National Health Research Institutes, Taiwan (NHRI-108A1- CACO13190902).
Declarations
Conflicts of interest
All authors declare no potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murdych TM. A case of severe autoimmune hemolytic anemia after a receipt of a first dose of SARS-CoV-2 vaccine. Int J Lab Hematol. 2021 doi: 10.1111/ijlh.13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulsen FO, Schaefers C, Langer F, Frenzel C, Wenzel U, Hengel FE, et al. Immune thrombocytopenic purpura after vaccination with COVID-19 vaccine (ChAdOx1 nCov-19) Blood. 2021;138(11):996–999. doi: 10.1182/blood.2021012790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Townsley DM, Scheinberg P, Winkler T, Desmond R, Dumitriu B, Rios O, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376(16):1540–1550. doi: 10.1056/NEJMoa1613878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnini I, Scappini B, Guidi S, Longo G, Bosi A. Acquired severe aplastic anemia after H1N1 influenza virus vaccination successfully treated with allogeneic bone marrow transplantation. Ann Hematol. 2012;91(3):475–476. doi: 10.1007/s00277-011-1278-0. [DOI] [PubMed] [Google Scholar]
- 6.Shah C, Lemke S, Singh V, Gentile T. Case reports of aplastic anemia after vaccine administration. Am J Hematol. 2004;77(2):204. doi: 10.1002/ajh.20153. [DOI] [PubMed] [Google Scholar]
- 7.Cecchi N, Giannotta JA, Barcellini W, Fattizzo B. A case of severe aplastic anaemia after SARS-CoV-2 vaccination. Br J Haematol. 2021 doi: 10.1111/bjh.17947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabata S, Hosoi H, Murata S, Takeda S, Mushino T, Sonoki T. Severe aplastic anemia after COVID-19 mRNA vaccination: Causality or coincidence? J Autoimmun. 2022;126:102782. doi: 10.1016/j.jaut.2021.102782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young NS. Aplastic anemia. N Engl J Med. 2018;379(17):1643–1656. doi: 10.1056/NEJMra1413485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pozzetto B, Legros V, Djebali S, Barateau V, Guibert N, Villard M, et al. Immunogenicity and efficacy of heterologous ChAdOx1-BNT162b2 vaccination. Nature. 2021;600(7890):701–706. doi: 10.1038/s41586-021-04120-y. [DOI] [PubMed] [Google Scholar]
- 11.Smith JN, Kanwar VS, MacNamara KC. Hematopoietic stem cell regulation by Type I and II interferons in the pathogenesis of acquired aplastic anemia. Front Immunol. 2016;7:330. doi: 10.3389/fimmu.2016.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]