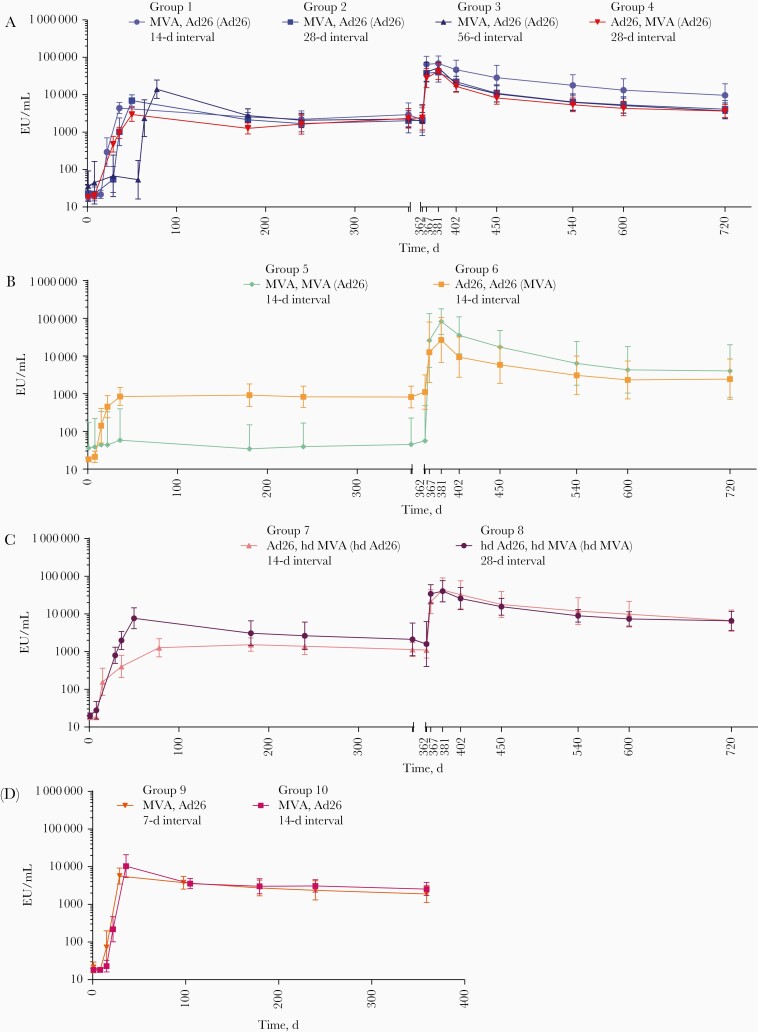
Figure 2.
Anti–Ebola virus glycoprotein immunoglobulin G binding antibody responses after heterologous or homologous 2-dose vaccination with Ad26.ZEBOV (adenovirus serotype 26 [Ad26]) and/or MVA-BN-Filo (modified vaccinia Ankara [MVA]) and booster vaccination at day 360 for groups 1–4 (A), 5 and 6 (B), 7 and 8 (C), and 9 and 10 (no booster vaccinations) (D). Vaccination regimens are shown as “dose 1, dose 2 (booster).” Responses represent geometric mean concentrations with 95% confidence intervals. Abbreviations: EU, enzyme-linked immunosorbent assay units; hdAd26, high-dose Ad26; hdMVA, high-dose MVA.